- Chlorpromazine
-
Chlorpromazine 
Systematic (IUPAC) name 3-(2-chloro-10H-phenothiazin-10-yl)-N,N-dimethyl-propan-1-amine Clinical data AHFS/Drugs.com monograph MedlinePlus a682040 Pregnancy cat. C—only when benefit for the mother exceeds risk to unborn child Legal status ℞ Prescription only Routes Oral, rectal (suppository), IM, IV infusion Pharmacokinetic data Bioavailability Oral, 30 to 50% (interindividual variations 10–70%) Metabolism Hepatic, mostly CYP2D6-mediated Half-life 16 to 30 hours. In long term treatment, CPZ induces its own metabolism Excretion Biliary and renal, as metabolites (only traces of unchanged drug) Identifiers CAS number 50-53-3  (free base)
(free base)
69-09-0 (hydrochloride)ATC code N05AA01 PubChem CID 2726 IUPHAR ligand 83 DrugBank DB00477 ChemSpider 2625 
UNII U42B7VYA4P 
KEGG D00270 
ChEBI CHEBI:3647 
ChEMBL CHEMBL71 
Chemical data Formula C17H19ClN2S Mol. mass 318.86 g/mol (free base)
355.33 g/mol (hydrochloride)SMILES eMolecules & PubChem  (what is this?) (verify)
(what is this?) (verify)Chlorpromazine (as chlorpromazine hydrochloride, abbreviated CPZ; marketed in the United States as Thorazine and elsewhere as Largactil) is a typical antipsychotic.[1] First synthesized on December 11, 1950, chlorpromazine was the first drug developed with specific antipsychotic action, and would serve as the prototype for the phenothiazine class of drugs, which later grew to comprise several other agents. The introduction of chlorpromazine into clinical use has been described as the single greatest advance in psychiatric care, dramatically improving the prognosis of patients in psychiatric hospitals worldwide; the availability of antipsychotic drugs curtailed indiscriminate use of electroconvulsive therapy and psychosurgery[citation needed], and was one of the driving forces behind the deinstitutionalization movement.
Chlorpromazine works on a variety of receptors in the central nervous system, producing anticholinergic, antidopaminergic, antihistaminic, and weak antiadrenergic effects. Both the clinical indications and side effect profile of CPZ are determined by this broad action: its anticholinergic properties cause constipation, sedation, and hypotension, and help relieve nausea. It also has anxiolytic (anxiety-relieving) properties. Its antidopaminergic properties can cause extrapyramidal symptoms such as akathisia (restlessness, aka the 'largactil shuffle' where the patient walks almost constantly, despite having nowhere to go due to mandatory confinement, and takes small, shuffling steps) and dystonia. It is known to cause tardive dyskinesia, which can be irreversible.[2] In recent years, chlorpromazine has been largely superseded by the newer atypical antipsychotics, which are usually better tolerated, and its use is now restricted to fewer indications. In acute settings, it is often administered as a syrup, which has a faster onset of action than tablets, and can also be given by intramuscular injection. IV administration is very irritating and is not advised; its use is limited to severe hiccups, surgery, and tetanus.[3]
Contents
History
 Advertisement for Thorazine (chlorpromazine) from the early 1960s[4]
Advertisement for Thorazine (chlorpromazine) from the early 1960s[4]
In 1933, the French pharmaceutical company Laboratoires Rhône-Poulenc began to search for new anti-histamines. In 1947, it synthesized promethazine, a phenothiazine derivative, which was found to have more pronounced sedative and antihistaminic effects than earlier drugs.[5] A year later, the French surgeon Pierre Huguenard used promethazine together with pethidine as part of a lytic cocktail to induce relaxation and indifference in surgical patients. Another surgeon, Henri Laborit, believed the compound stabilized the central nervous system by causing 'artificial hibernation', and described this state as 'sedation without narcosis'. He suggested to Rhône-Poulenc that they develop a compound with better stabilizing properties.[6] The chemist Paul Charpentier produced a series of compounds and selected the one with the least peripheral activity, known as RP4560 or chlorpromazine, on 11 December 1950. Simone Courvoisier conducted behavioural tests and found chlorpromazine produced indifference to aversive stimuli in rats. Chlorpromazine was distributed for testing to physicians between April and August 1951. Laborit trialled the medicine on at the Val-de-Grâce military hospital in Paris, using it as an anaesthetic booster in intravenous doses of 50 to 100 mg on surgery patients and confirming it as the best drug to date in calming and reducing shock, with patients reporting improved well being afterwards. He also noted its hypothermic effect and suggested it may induce artificial hibernation. Laborit thought this would allow the body to better tolerate major surgery by reducing shock, a novel idea at the time. Known colloquially as "Laborit's drug", chlorpromazine was released onto the market in 1953 by Rhône-Poulenc and given the trade name Largactil, derived from large "broad" and acti* "activity.[7]
Following on, Laborit considered whether chlorpromazine may have a role in managing patients with severe burns, Raynaud's phenomenon, or psychiatric disorders. At the Villejuif Mental Hospital in November 1951, he and Montassut administered an intravenous dose to psychiatrist Cornelio Quarti who was acting as a volunteer. Quarti noted the indifference, but fainted upon getting up to go to the toilet, and so further testing was discontinued. Despite this, Laborit continued to push for testing in psychiatric patients during early 1952. Psychiatrists were reluctant initially, but on January 19, 1952, it was administered (alongside pethidine, penthothal and ECT) to Jacques Lh. a 24 year old manic patient, who responded dramatically, and was discharged after three weeks having received 855 mg of the drug in total.[7]
Pierre Deniker had heard about Laborit's work from his brother in law, who was a surgeon, and ordered chlorpromazine for a clinical trial at the Hôpital Sainte-Anne in Paris where he was Men's Service Chief.[7] Together with the Director of the hospital, Professor Jean Delay, they published first clinical trial in 1952, in which they treated 38 psychotic patients with daily injections of chlorpromazine without the use of other sedating agents.[8] The response was dramatic; treatment with chlorpromazine went beyond simple sedation with patients showing improvements in thinking and emotional behaviour.[1] They also found that doses higher than those used by Laborit were required, giving patients 75–100 mg daily.[7]
Deniker then visited America, where the publication of their work alerted the American psychiatric community that the new treatment might represent a real breakthrough. Heinz Lehmann of the Verdun Protestant Hospital in Montreal trialled it in 70 patients and also noted its striking effects, with patients' symptoms resolving after many years of unrelenting psychosis[citation needed]. By 1954, chlorpromazine was being used in the United States to treat schizophrenia, mania, psychomotor excitement, and other psychotic disorders.[9][10][11] Rhône-Poulenc licensed chlorpromazine to Smith Kline & French (today's GlaxoSmithKline) in 1953. In 1955 it was approved in the United States for the treatment of emesis (vomiting). The effect of this drug in emptying psychiatric hospitals has been compared to that of penicillin and infectious diseases.[8] But the popularity of the drug fell from the late 1960s as newer drugs came on the scene. From chlorpromazine a number of other similar antipsychotics were developed. It led to the discovery of antidepressants.[12]
Chlorpromazine largely replaced electroconvulsive therapy, psychosurgery, and insulin shock therapy.[1] By 1964, about 50 million people worldwide had taken it.[13] The development and use of antipsychotic drugs like chlorpromazine was one of the forces that propelled deinstitutionalization, the systematic removal of people with severe mental illness from institutions like psychiatric hospitals and their reintegration into the community[citation needed]. In 1955 there were 558,922 resident patients in American state and county psychiatric hospitals. By 1970, the number dropped to 337,619; by 1980 to 150,000, and by 1990 between 110,000 and 120,000 patients.[14]
Chlorpromazine, in widespread use for 50 years, remains a "benchmark" drug in the treatment of schizophrenia, an effective drug although not perfect.[15][16] The relative strengths or potencies of other antipsychotics are often ranked or measured against chlorpromazine in aliquots of 100 mg, termed chlorpromazine equivalents or CPZE.[17]
Indications
Chlorpromazine is classified as a low-potency typical antipsychotic and in the past was used in the treatment of both acute and chronic psychoses, including schizophrenia and the manic phase of bipolar disorder as well as amphetamine-induced psychoses. Low-potency antipsychotics have more anticholinergic side effects such as dry mouth, sedation and constipation, and lower rates of extrapyramidal side effects, while high-potency antipsychotics (such as haloperidol) have the reverse profile[citation needed].
The use of chlorpromazine and other typical antipsychotics has been largely replaced by newer generation of atypical antipsychotics which are generally better tolerated[citation needed]. Recent global review of data supports its effectiveness as an antipsychotic.[15][18]
Chlorpromazine has also been used in porphyria and as part of tetanus treatment. It still is recommended for short term management of severe anxiety and aggressive episodes. Resistant and severe hiccups, severe nausea/emesis and preanesthetic conditioning are other uses.[18][19] Symptoms of delirium in medically hospitalized AIDS patients have been effectively treated with low doses of chlorpromazine.[20]
Off-label and controversial uses
Chlorpromazine is occasionally used off-label for treatment of severe migraine. Sometimes it is used in small doses to improve the nausea that opioid-treated cancer patients encounter and to intensify and prolong the analgesic action of the opioids given. It remains controversial whether or not chlorpromazine has its own analgesic properties. Analgesic properties may result from a central action on the hypothalamus; the patient may feel pain much less than before. Other mechanisms may be an interaction with opioid receptors centrally and/or in the spinal cord. Some experts even say that chlorpromazine, like other phenothiazines, may even have antianalgesic properties.
It has a unique action in cholera, reducing the loss of water by approximately 30 percent.
In Germany, chlorpromazine still carries label indications for insomnia and severe pruritus, as well as preanesthesia.[21]
Veterinary uses
Chlorpromazine is not registered for animal uses, but may be prescribed legally by veterinarians for animal use. It is primarily used as an antiemetic in dogs and cats, and it is commonly used to decrease nausea in animals that are too young for other common anti-emetics. It is also sometimes used as a preanesthetic and muscle relaxant in cattle, swine, sheep, and goats[citation needed]. It is generally contraindicated for use with horses, due to a high incidence of ataxia and altered mentation. Its use in food-producing animals has been banned in the EU according to the Council's regulation 2377/90.
Adverse effects
The main side effects of chlorpromazine are due to its anticholinergic properties; these effects overshadow and counteract, to some extent, the extrapyramidal side effects typical of many early generation antipsychotics. These include sedation, slurred speech, dry mouth, constipation, urinary retention and possible lowering of seizure threshold. Appetite may be increased with resultant weight gain, and Glucose tolerance may be impaired.[22] It lowers blood pressure with accompanying dizziness.[15] Memory loss and amnesia has also been reported. Chlorpromazine, which has sedating effects, will increase sleep time when given at high doses or when first administered, although tolerance usually develops.[23] Sleep cycles or REM sleep is not altered by antipsychotics.[24]
Dermatological reactions are frequently observed. In fact three types of skin disorders are observed: hypersensitivity reaction, contact dermatitis, and photosensitivity. During long-term therapy of schizophrenic patients chlorpromazine can induce abnormal pigmentation of the skin. This can be manifested as gray-blue pigmentation in regions exposed to sunlight.[23]
There are adverse effects on the reproductive system. Phenothiazines are known to cause hyperprolactinaemia leading to amenorrhea, cessation of normal cyclic ovarian function, loss of libido, occasional hirsutism, false positive pregnancy tests, and long-term risk of osteoporosis in women. The effects of hyperprolactinemia in men are gynaecomastia, lactation, impotence, loss of libido, and hypospermatogenesis. These antipsychotics have significant effects on gonadal hormones including significantly lower levels of estradiol and progesterone in women whereas men display significantly lower levels of testosterone and DHEA when undergoing antipsychotic drug treatment compared to controls.[25] According to one study of the effects on the reproductive system in rats treated with chlorpromazine there were significant decreases in the weight of the testis, epididymis, seminal vesicles, and prostate gland. This was accompanied by a decline in sperm motility, sperm counts, viability, and serum levels of testosterone in chlorpromazine rats compared to control rats. It has been reported that a change in either the absolute or relative weight of an organ after a chemical is administered is an indication of the toxic effect of the chemical. Therefore, the observed change in the relative weight of the testis and other accessory reproductive organs in rats treated with chlorpromazine indicates that the drug might be toxic to these organs at least during the period of treatments. Furthermore, the weights of the kidney, heart, liver, and adrenal glands of these treated rats were not affected both during administration of the drug and recovery periods, suggesting that the drug is not toxic to these organs.[25]
Antipsychotic drugs may cause priapism, a pathologically prolonged and painful penile erection, which is usually unassociated with sexual desire or intercourse. Although this effect is rare it is a potentially serious complication that can lead to permanent impotence and other serious complications.[26]
Even therapeutically low doses may trigger seizures in susceptible patients, such as those with an abnormally low genetically determined seizure threshold, presumably by lowering the seizure threshold. The incidence of the first unprovoked seizure in the general population is from 0.07 to 0.09%, but in patients treated with commonly used antipsychotic drugs it reportedly ranges from 0.1 to 1.5%. In overdose, the risk reaches 4 to 30%. This wide variability among studies may be due to methodological differences. The risk is greatly influenced by the individual's inherited seizure threshold, and particularly by a history of epilepsy, brain damage or other conditions. The triggering of seizures by antipsychotic drugs is generally agreed to be a dose-dependent adverse effect.[27]
Tardive dyskinesia and akathisia are less commonly seen with chlorpromazine than they are with high potency typical antipsychotics such as haloperidol[28] or trifluoperazine, and some evidence suggests that, with conservative dosing, the incidence of such effects for chlorpromazine may be comparable to that of newer agents such as risperidone or olanzapine.[29]
A particularly severe side effect is neuroleptic malignant syndrome, which can be fatal.[30] Other reported side effects are rare, though severe; these include a reduction in the number of white blood cells—referred to as leukopenia—or, in extreme cases, even agranulocytosis, which may occur in 0.01% of patients and lead to death via uncontrollable infections and/or sepsis. Chlorpromazine is also known to accumulate in the eye—in the posterior corneal stroma, lens, and uveal tract. Because it is a phototoxic compound, the potential exists for it to cause cellular damage after light exposure. Research confirms a significant risk of blindness from continued use of chlorpromazine, as well as other optological defects such as color blindness and benign pigmentation of the cornea.[31]
Cardiotoxic effects of phenothiazines in overdose are similar to that of the tricyclic antidepressants.[23] Cardiac arrhythmia and apparent sudden death have been associated with therapeutic doses of chlorpromazine, however they are rare cases. The sudden cardiovascular collapse is attributable to ventricular dysrhythmia. Supraventricular tachycardia may also develop. Patients on chlorpromazine therapy exhibit abnormalities on the electrocardiographic T and U waves. These major cardiac arrhythmias that are lethal are a potential hazard even in patients without heart disease who are receiving therapeutic doses of antipsychotic drugs. In order to quantify the risk of cardiac complications to patients receiving therapeutic doses of phenothiazines a prospective clinical trial is suggested.[32]
Interactions
Chlorpromazine intensifies the central depressive action of drugs with such activity (such as tranquilizers, barbiturates, narcotics, antihistamines, OTC antiemetics). It also intensifies the actions and undesired side effects of antihypertensive and anticholinergic drugs. The combination of chlorpromazine with other antipsychotics may result in increased central depression, hypotension and extrapyramidal side effects, but may enhance the clinical results of therapy. The anti-worm drug (antihelminthic) piperazine may intensify extrapyramidal side effects. In general, all antipsychotics may lead to seizures in combination with tramadol (Ultram). Chlorpromazine may increase the insulin needs of diabetic patients.[33][34] Chorpromazine enhances the CNS depressant effects of alcohol.[35]
Chlorpromazine is able to inhibit dextromethorphan 0-demethylation, a selective marker for CYP2D6, in a concentration dependent manner. It inhibits the catalytic activity of cytochrome P450 isoforms. CYP2D6 enzyme is not only important in the metabolism of chlorpromazine and associated antipsychotics but is also important in the metabolism of tricyclic antidepressants and selective serotonin reuptake inhibitors that are commonly prescribed to patients with psychiatric disorders. This may result in significant drug interactions which may put these people at a heightened risk for side effects that may be masked by the positive effects or side effects of antipsychotic drugs themselves. Therefore, drugs that inhibit the enzymes that metabolize chlorpromazine would be expected to cause increases in the concentration of other antipsychotic drugs that are co-administered. These increases in concentration may in turn lead to the development of antipsychotic-induced side effects, developing pharmacokinetic interactions with other antipsychotics and antidepressant drugs that are coadministered.[36] Differential expression of various CYP isoforms in specific brain locations leads to the conclusion that antipsychotic drugs could be metabolized to different products in different regions of the brain. The varying levels of expression of the CYP isoforms between individuals and for each particular antipsychotic as well as the possibility of differential metabolism in the brain provides one possible reason why there is such a wide range of adverse effects and therapeutic effects of chlorpromazine and the other antipsychotic drugs in the population of patients currently using.[36]
As chlorpromazine can enhance the central nervous system depression produced by other CNS depressant drugs, its administration with alcohol results in increased sedative effects and impaired co-ordination. An interaction between phenothiazine and caffeinated beverages has been reported. Addition of coffee or tea to phenothiazine or butyrophenone antipsychotics forms a precipitant in vitro. This finding was of initial concern as many psychiatric patients might drink coffee or tea immediately after receiving oral medication. However in humans caffeine use was only slightly related to antipsychotic levels. These negative findings between in vitro studies and human epidemiological studies can be attributable to the stomach acidity which reverses any precipitation. It still remains unclear whether the caffeine-antipsychotic precipitation phenomenon has any clinical significance. The neurophysiology of this relationship is derived from the fact that cytochrome P450 CYP1A2 isoenzyme is responsible for metabolism of caffeine as well as chlorpromazine, thus they may compete for the isoenzyme. Support of this possible competition of the isoenzyme comes from the observation that high doses of caffeine can cause tremors and restless legs both of which could be mistaken for or could aggravate neuroleptic induced extrapyramidal effects.[37]
Chlorpromazine has been shown to inhibit the human ether-a-go-go related gene (hERG) potassium channels. This is a serious side effect of the drug and could lead to death. When this occurs long QT syndrome (aLQTS) is acquired by the prolongation of the cardiac action potential due to a block in the cardiac ion channels and delayed repolarization of the heart. Patients with aLQTS are exposed to a higher chance of torsade de pointes arrhythmias and sudden cardiac arrest.[38]
Chlorpromazine is notorious for depositing ocular tissues when taken in high dosages for long periods of time. In one specific case a 59 year old schizophrenic man on chlorpromazine therapy with cumulative dosage of 2500 g resulted in multiple white deposits in the endothelium of both corneas. Confocal microscopy revealed significant pleomorphism and polymegethism of endothelial cells. The anterior lens capsules opacities were star- shaped and concentrated in the centre. In this patient chlorpromazine deposited mainly in the corneal endothelium, central anterior lens capsule and epithelial cells. This is common with many patients that receive high dosages of chlorpromazine.[39]
Pharmacology
Pharmacokinetics
Chlorpromazine, and many other phenothiazine derivatives, are highly lipophilic molecules that readily bind with membranes and proteins. Around 95-98% of the drug is bound in the plasma; 85% of the drug is bound to the plasma protein albumin. Renal disease may cause this range to expand significantly.[40] Highest concentrations of unconjugated chlorpromazine metabolites are found in the lungs and the liver.[23] It is a dopamine inhibitor, increases dopamine turnover in the brain, and stimulates prolactin release. The increased brain turnover of dopamine may be related to its therapeutic effects[citation needed]; it achieves a higher concentration in the brain than in the blood stream.[23]
The drug can also enter fetal circulation and breast milk, so pregnant and nursing mothers must beware, especially since fetuses have a low rate of phenothiazine metabolism. With gas chromatography, levels of chlorpromazine and some of its metabolites can be measured in the milk and plasma of nursing mothers. In one case study of nursing mothers on chlorpromazine therapy, the drug itself was detected in milk samples and ranged from 7 ng/ml to 98 ng/ml. The metabolite chlorpromazine sulphoxide was present in all samples. Plasma levels of CPZ ranged from 16 ng/ml to 52 ng/ml. However, there was no clear or consistent relationship between plasma and milk levels of CPZ. In one mother who did in fact feed her baby her breast milk, the milk CPZ level was 92 ng/ml and the baby was reported to be drowsy and lethargic. Therefore, there should be some caution in allowing nursing mothers currently on CPZ therapy and presumably related antipsychotics to breast feed their children.[41]
Chlorpromazine is able to cross the placental barrier, and it has been shown that drug doses higher than 500 mg daily in late pregnancy are associated with an increased incidence of respiratory distress in newborns. One case study reported that a newborn who was not breast fed but was exposed to CPZ in utero had detectably large amounts of the drug in its urine. This indicated the drug can in fact cross the placental barrier and is slowly cleared out of the body due to the infant's immature liver. Pregnant women and nursing mothers should thus be advised of the effects of CPZ on their newborn's health.[42]
Bioavailability: Only about 32% of the administered dose is available to the systemic circulation in the active form. Over time and multiple administrations, bioavailability may drop to 20%. Peak concentrations are achieved in 1 to 4 hours[9] (range 1.5–8 hours), after an oral dose.[43]
Chlorpromazine is derived from phenothiazine, has an aliphatic side chain, typical for low to middle potency antipsychotics. Chlorpromazine is slowly absorbed from the intramuscular injection site with the peak plasma concentration occurring 6–24 hours after administration of the drug. The oral bioavailability is estimated to be 30–50% that of intramuscular doses and about 10% that of intravenous doses due to extensive first pass metabolism in the liver. Its elimination half-life is 16–30 hours (8–35 hours, although it is as short as 2 hours or as long as 60 hours in some individuals),[43] due to high lipophilicity, high membrane-binding, and high protein-binding. It has many active metabolites (more than 100 metabolites being theoretically possible) with greatly varying halflives and pharmacological profiles.A number of the metabolites may contribute to the pharmacological effects of chlorpromazine including 7-hydroxychlorpromazine, chlorpromazine-N-oxide, 3-hydroxychlorpromazine and desmethylchlorpromazine.)[43] Although the metabolite chlorpromazine-N-oxide does not possess activity in vitro, it can exert an indirect pharmacological effect in vivo by reverting back to chlorpromazine. The major routes of metabolism include hydroxylation, N-oxidation, sulphoxidation, demethylation, deamination and conjugation. There is little evidence supporting the development of metabolic tolerance or an increase in the metabolism of chlorpromazine due to microsomal liver enzymes following multiple doses of the drug.[44] The mechanism of action of chlorpromazine is that the drug can act as an uncoupling agent of oxidative phosphorylation and also as an inhibitor of ATP-ase and cytochrome oxidase. However, the relationship that may exist between these mechanisms are not entirely understood.
The cytochrome P450 isoenzymes 1A2 and 2D6 are needed for metabolism of chlorpromazine. CYP 2D6 is the main enzyme catalyzing 7-hydroxylation of chlorpromazine, the reaction being partially catalyzed by CYP 1A2.[37]
Chlorpromazine is typically degraded by the liver by the action of cytochrome-P450 family enzymes, usually CYP2D6. Less than 1% of the unchanged drug is excreted via the kidneys in the urine. In which 20-70% is excreted as conjugated or unconjugated metabolites, whereas 5-6% is excreted in feces.[43] There are on the order of 10 or more major metabolites generated by the hepatic pathway in appreciable concentrations. The three most common appear in the following image. The first is the doubly N-demethylated species, followed by the 7-hydroxylated form, and finally chlorophenothiazine, in which the entire R1 side chain is missing.[45]
Often, due to their high lipophilic character, these and other metabolites may be detected in the urine up to 18 months.[43] after discontinuation of use. Most metabolites lack any sort of antipsychotic activity, but a few are biologically active. These include 7-hydroxychlorpromazine, mesoridazine, and a few N-demethylated metabolites.[9]
Pharmacodynamics and central effects
Chlorpromazine is a very effective antagonist of D2 dopamine receptors and similar receptors, such as D3 and D5. Unlike most other drugs of this genre, it also has a high affinity for D1 receptors. Blocking these receptors causes diminished neurotransmitter binding in the forebrain, resulting in many different effects. Dopamine, unable to bind with a receptor, causes a feedback loop that causes dopaminergic neurons to release more dopamine. Therefore, upon first taking the drug, patients will experience an increase in activity of dopaminergic neural activity. Eventually, dopamine production of the neurons will drop substantially and dopamine will be removed from the synaptic cleft. At this point, neural activity decreases greatly; the continual blockade of receptors only compounds this effect.[9]
Chlorpromazine acts as an antagonist (blocking agent) on different postsynaptic receptors:
- dopamine receptors (subtypes D1, D2, D3 and D4), which account for its different antipsychotic properties on productive and unproductive symptoms;in the mesolimbic dopamine system accounts for the antipsychotic effect whereas the blockade in the nigrostriatal system produces the extrapyramidal effects
- serotonin receptors (5-HT1 and 5-HT2), with anxiolytic, and antiaggressive properties as well as an attenuation of extrapyramidal side effects, but also leading to weight gain, fall in blood pressure, sedation and ejaculation difficulties,
- histamine receptors (H1 receptors, accounting for sedation, antiemetic effect, vertigo, fall in blood pressure and weight gain),
- α1- and α2-adrenergic receptors (antisympathomimetic properties, lowering of blood pressure, reflex tachycardia, vertigo, sedation, hypersalivation and incontinence as well as sexual dysfunction, but may also attenuate pseudoparkinsonism—controversial), and
- M1 and M2 muscarinic acetylcholine receptors (causing anticholinergic symptoms such as dry mouth, blurred vision, constipation, difficulty or inability to urinate, sinus tachycardia, electrocardiographic changes and loss of memory, but the anticholinergic action may attenuate extrapyramidal side effects).
The presumed effectiveness of the antipsychotic drugs relied on their ability to block dopamine receptors. This assumption arose from the dopamine hypothesis that maintains that both schizophrenia and bipolar disorder are a result of excessive dopamine activity. Furthermore, psychomotor stimulants like cocaine that increase dopamine levels can cause psychotic symptoms if taken in excess.[46]
Chlorpromazine and other typical antipsychotics are primarily blockers of D2 receptors. In fact an almost perfect correlation exists between the therapeutic dose of a typical antipsychotic and the drug's affinity for the D2 receptor. Therefore, a larger dose is required if the drug’s affinity for the D2 receptor is relatively weak. A correlation exists between average clinical potency and affinity of the antipsychotics for dopamine receptors.[24] Chlorpromazine tends to have greater effect at serotonin receptors than at D2 receptors, which is notably the opposite effect of the other typical antipsychotics. Therefore, chlorpromazine with respect to its effects on dopamine and serotonin receptors is similar to the atypical antipsychotics than the typical antipsychotics.[24]
Chlorpromazine and other antipsychotics with sedative properties such as promazine and thioridazine are among the most potent agents at α-adrenergic receptors. Furthermore, they are also among the most potent antipsychotics at histamine H1 receptors. This finding is in agreement with the pharmaceutical development of chlorpromazine and other antipsychotics as anti-histamine agents. Furthermore, the brain has a higher density of histamine H1 receptors than any body organ examined which may account for why chlorpromazine and other phenothiazine antipsychotics are as potent at these sites as the most potent classical antihistamines.[47]
In addition to influencing the neurotransmitters dopamine, serotonin, epinephrine, norepinephrine, and acetylcholine it has been reported that antipsychotic drugs could achieve glutamatergic effects. This mechanism involves direct effects on antipsychotic drugs on glutamate receptors. By using the technique of functional neurochemical assay chlorpromazine and phenothiazine derivatives have been shown to have inhibitory effects on NMDA receptors that appeared to be mediated by action at the Zn site. It was found that there is an increase of NMDA activity at low concentrations and suppression at high concentrations of the drug. No significant difference in glutamate and glycine activity from the effects of chlorpromazine were reported. Further work will be necessary to determine if the influence in NMDA receptors by antipsychotic drugs contributes to their effectiveness.[48]
Peripheral effects
Chlorpromazine is an antagonist to H1 receptors (provoking antiallergic effects), H2 receptors (reduction of forming of gastric juice), M1 and M2 receptors (dry mouth, reduction in forming of gastric juice) and some 5-HT receptors (different anti-allergic/gastrointestinal actions).
Because it acts on so many receptors, chlorpromazine is often referred to as a "dirty drug",[49] whereas the atypical antipsychotic amisulpride, for example, acts only on central D2 and D3 receptors and is therefore a "clean drug". Research still needs to be done to understand the implications of this fact.
Tolerance and withdrawal
The British National Formulary recommends a gradual withdrawal when discontinuing antipsychotic treatment to avoid acute withdrawal syndrome or rapid relapse.[50] While withdrawal symptoms can occur, there is no evidence that tolerance develops to the drug's antipsychotic effects. A patient can be maintained for years on a therapeutically effective dose without any decrease in effectiveness being reported. Tolerance appears to develop to the sedating effects of chlorpromazine when it is first administered. Tolerance also appears to develop to the extrapyramidal, parkinsonian and other neuroleptic effects, although this is debatable.[24]
A failure to notice withdrawal symptoms may be due to the relatively long half life of the drug resulting in the extremely slow excretion from the body. However, there are reports of muscular discomfort, exaggeration of psychotic symptoms and movement disorders, and difficulty sleeping when the antipsychotic drug is suddenly withdrawn, but after years of normal doses these effects are not normally seen.[24]
Dosage and administration
A wide range is covered from 25 mg oral or intramuscular for mild sedation, every 8 hours, up to 100 mg every 6 hours for severely ill patients.[18] Initial doses should be low and be increased gradually. It is recommended that most of the daily dose is given at bedtime for maximum hypnotic activity and minimal daytime sedation and hypotension. In the US there are controlled release forms of chlorpromazine (e.g. 300 mg). After the individual dose is well established, such a CR capsule can be given with the evening meal as a single dose, covering the next 24 hours. It is often administered in acute settings as a syrup, which has a faster onset of action than tablets,[18][22] and is more difficult to spit out to avoid taking.[citation needed]
Chlorpromazine and other antipsychotic drugs need to be taken long-term to prevent the symptoms of the disease from reappearing. Abuse of antipsychotics is unlikely due to their unpleasant effects, in fact these effects often lead patients to stop taking them. For this reason various administration techniques have been developed that do not depend on a patient's compliance. Among them is administration of depot injections which slowly releases the drug and maintains the appropriate blood levels. The technique involves an IM dose injected into a muscle (usually the gluteus medius) of the buttocks or deltoid muscle in the shoulder. The drug slowly diffuses into the body fluids. A single depot injection of an antipsychotic drug can be effective for as long as four weeks.[24] Chlorpromazine is not available as a depot medication.
Previously, higher doses, up to 1200 mg daily or more, were used in acute psychosis. However, this range has markedly decreased in recent years, and dosing aims for the lowest possible with good therapeutic effect. Dosage in ambulatory patients should be particularly low (minimizing sedation and hypotension). Intravenous injection of undiluted solution is contraindicated due to risk of massive fall in blood pressure, cardiovascular collapse. For intravenous infusion of dilutions, the (hospitalized) patient should be lying and the infusion rate should be as slow as possible. Afterward the patient should rest in the lying position for at least 30 minutes.[19]
All patients treated with chlorpromazine on a long-term-basis should have the following checked regularly: blood-pressure, pulse rate, laboratory-tests (liver function tests, kidney-values, blood cell counts, ECG and EEG. Some side effects seem to appear more frequently during the first months of therapy (sedation, hypotension, liver damage) while others such as tardive dyskinesia can appear over time.
Discontinuation
At regular intervals the treating physician should evaluate whether continued treatment is needed. The drug should never be discontinued suddenly, due to unpleasant withdrawal-symptoms, such as agitation, sleeplessness, states of anxiety, stomach pain, dizziness, nausea and vomiting. Preferably the dose should be gradually reduced.[19]
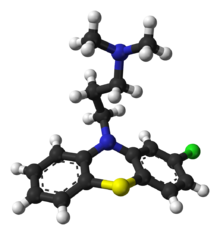
Synthesis
The synthesis of chlorpromazine begins with the reaction of 1,4-dichloro-2-nitrobenzene with 2-bromobenzenethiol.[51][52][53] Hydrogen chloride is evolved as a by-product of this step and a thioether is formed as the product. Although not verified, it appears that the ortho-chlorine is eliminated preferentially. In the second step the nitrogroup is reduced with hydrogen gas. Upon heating in DMF solvent, ring cyclization occurs. In an analogous manner to what was done in the preparation of promazine, the 2-chloro-10H-phenothiazine of the last step is combined with 3-chloro-N,N-dimethylpropan-1-amine in the presence of sodamide base.
References
- ^ a b c Healy, David (2004). The Creation of Psychopharmacology. Harvard University Press. pp. 37–73. ISBN 9780674015999. http://books.google.com/?id=6O2rPJnyhj0C&printsec=frontcover&dq=isbn=9780674015999&cd=1#v=onepage&q=chlorpromazie. Retrieved 6 July 2010.
- ^ Diaz, Jaime (1997). How drugs influence behavior: a neuro behavioral approach. Englewood Cliffs, N.J: Prentice Hall. p. 285. ISBN 978-0-02-328764-0.
- ^ "Chlorpromazine - Thorazine Dilution Guidelines". GlobalRPh Inc.. http://www.globalrph.com/chlorpromazine_dilution.htm. Retrieved 19 February 2010.
- ^ The text reads: When the patient lashes out against "them" - THORAZINE (brand of chlorpromazine) quickly puts an end to his violent outburst. 'Thorazine' is especially effective when the psychotic episode is triggered by delusions or hallucinations. At the outset of treatment, Thorazine's combination of antipsychotic and sedative effects provides both emotional and physical calming. Assaultive or destructive behavior is rapidly controlled. As therapy continues, the initial sedative effect gradually disappears. But the antipsychotic effect continues, helping to dispel or modify delusions, hallucinations and confusion, while keeping the patient calm and approachable. SMITH KLINE AND FRENCH LABORATORIES leaders in psychopharmaceutical research.
- ^ Healy, David (2004). "Explorations in a new world". The creation of psychopharmacology. Harvard University Press. p. 77. ISBN 978-0-674-01599-9. http://books.google.com/?id=6O2rPJnyhj0C&printsec=frontcover&dq=isbn=9780674015999&cd=1#v=onepage&q.
- ^ Healy, David (2004). "Explorations in a new world". The creation of psychopharmacology. Harvard University Press. p. 80. ISBN 978-0-674-01599-9.
- ^ a b c d López-Muñoz, Francisco; Alamo, Cecilio; Cuenca, Eduardo; Shen, Winston W.; Clervoy, Patrick; Rubio, Gabriel (2005). "History of the discovery and clinical introduction of chlorpromazine". Annals of Clinical Psychiatry 17 (3): 113–35. doi:10.1080/10401230591002002. PMID 16433053.
- ^ a b Turner T (January 2007). "Chlorpromazine: unlocking psychosis". BMJ 334 (Suppl 1): s7. doi:10.1136/bmj.39034.609074.94. PMID 17204765.
- ^ a b c d Gilman, Alfred; Goodman, Louis Sanford; Hardman, Joel G.; Limbird, Lee E., ed (2001). Goodman & Gilman's the pharmacological basis of therapeutics (10th ed.). New York: McGraw-Hill. pp. 447–449. ISBN 978-0-07-135469-1.
- ^ Long, James W. (1992). The Essential guide to prescription drugs. New York: HarperPerennial. pp. 321–325. ISBN 978-0-06-271534-0.
- ^ Reines, Brandon P (1990). "The Relationship between Laboratory and Clinical Studies in Psychopharmacologic Discovery". Perspectives on Medical Research (Medical Research Modernization Society) 2. http://www.curedisease.net/reports/Perspectives/vol_2_1990/PsycholDisc.html. Retrieved 2009-06-01.
- ^ Healy, David (2004). "Introduction". The Creation of Psychopharmacology. Harvard University Press. p. 2. ISBN 0674015991. http://books.google.com/?id=6O2rPJnyhj0C&printsec=frontcover&dq=isbn=9780674015999&cd=1#v=onepage&q=chlorpromazine.
- ^ "Drug for treating schizophrenia identified". pbs.org. http://www.pbs.org/wgbh/aso/databank/entries/dh52dr.html. Retrieved 7 July 2010.
- ^ McKenzie, James F.; Pinger, R. R.; Kotecki, Jerome Edward (2008). An introduction to community health. Boston: Jones and Bartlett Publishers. ISBN 0-7637-4634-7.[page needed]
- ^ a b c Adams CE, Awad G, Rathbone J, Thornley B (2007). Adams, Clive E. ed. "Chlorpromazine versus placebo for schizophrenia". Cochrane Database of Systematic Reviews (2): CD000284. doi:10.1002/14651858.CD000284.pub2. PMID 17443500.
- ^ Thornley B, Rathbone J, Adams CE, Awad G (2003). Thornley, Ben. ed. "Chlorpromazine versus placebo for schizophrenia". Cochrane Database of Systematic Reviews (2): CD000284. doi:10.1002/14651858.CD000284. PMID 12804394.
- ^ Yorston, G. (2000). "Chlorpromazine equivalents and percentage of British National Formulary maximum recommended dose in patients receiving high-dose antipsychotics". Psychiatric Bulletin 24 (4): 130. doi:10.1192/pb.24.4.130.
- ^ a b c d Gilman, Alfred; Goodman, Louis Sanford; Hardman, Joel G.; Limbird, Lee E., ed (2001). Goodman & Gilman's the pharmacological basis of therapeutics (10th ed.). New York: McGraw-Hill. pp. 499–506. ISBN 978-0-07-135469-1.
- ^ a b c American Society of Health-System Pharmacists (November 1, 2008). "Chlorpromazine". PubMed Health. National Center for Biotechnology Information. http://www.ncbi.nlm.nih.gov/pubmedhealth/PMH0000553.
- ^ Breitbart W, Marotta R, Platt MM et al. (February 1996). "A double-blind trial of haloperidol, chlorpromazine, and lorazepam in the treatment of delirium in hospitalized AIDS patients". The American Journal of Psychiatry 153 (2): 231–7. PMID 8561204. http://ajp.psychiatryonline.org/cgi/pmidlookup?view=long&pmid=8561204.
- ^ "Propaphenin, Medicine and Disease information". EPG Online. 2001-07-14. http://www.epgonline.org/viewdrug.cfm/letter/P/language/LG0017/drugId/DR007815/drugName/Propaphenin%C2%AE. Retrieved 2010-07-18.
- ^ a b "Chlorpromazine". www.mentalhealth.com. http://www.mentalhealth.com/drug/p30-c01.html. Retrieved 6 July 2010.
- ^ a b c d e Higa de Landoni, Julia. "Chlorpromazine". inchem.org. http://www.inchem.org/documents/pims/pharm/chlorpro.htm. Retrieved 7 July 2010.
- ^ a b c d e f McKim,, William A. (2007). Drugs and behavior: an introduction to behavioral pharmacology (6th ed.). Upper Saddle River, NJ: Prentice Hall. p. 416. ISBN 978-0-13-219788-5.
- ^ a b Raji Y, Ifabunmi SO, Akinsomisoye OS, Morakinyo AO, Oloyo AK (2005). "Gonadal Responses to Antipsychotic Drugs: Chlorpromazine and Thioridazine Reversibly Suppress Testicular Functions in Albino Rats". International Journal of Pharmacology 1 (3): 287–92. doi:10.3923/ijp.2005.287.292.
- ^ Wang CS, Kao WT, Chen CD, Tung YP, Lung FW (July 2006). "Priapism associated with typical and atypical antipsychotic medications". International Clinical Psychopharmacology 21 (4): 245–8. doi:10.1097/00004850-200607000-00008. PMID 16687997.
- ^ Pisani F, Oteri G, Costa C, Di Raimondo G, Di Perri R (2002). "Effects of psychotropic drugs on seizure threshold". Drug Safety 25 (2): 91–110. doi:10.2165/00002018-200225020-00004. PMID 11888352.
- ^ Leucht C, Kitzmantel M, Chua L, Kane J, Leucht S (2008). Leucht, Claudia. ed. "Haloperidol versus chlorpromazine for schizophrenia". Cochrane Database of Systematic Reviews (1): CD004278. doi:10.1002/14651858.CD004278.pub2. PMID 18254045.
- ^ Leucht S, Wahlbeck K, Hamann J, Kissling W (May 2003). "New generation antipsychotics versus low-potency conventional antipsychotics: a systematic review and meta-analysis". Lancet 361 (9369): 1581–9. doi:10.1016/S0140-6736(03)13306-5. PMID 12747876.
- ^ Mahmood T, Warren JP (May 1989). "Neuroleptic malignant syndrome from chlorpromazine: case report". The Journal of the Royal College of General Practitioners 39 (322): 211. PMC 1712026. PMID 2560008. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=1712026.
- ^ Legwold, Gary. "I Can See Clearly Now". Experience Life Magazine (Lifetime Fitness). http://www.lifetimefitness.com/magazine/index.cfm?strWebAction=article_detail&intArticleId=522. Retrieved 2006-06-02.
- ^ Fowler NO, McCall D, Chou TC, Holmes JC, Hanenson IB (February 1976). "Electrocardiographic changes and cardiac arrhythmias in patients receiving psychotropic drugs". The American Journal of Cardiology 37 (2): 223–30. doi:10.1016/0002-9149(76)90316-7. PMID 2004.
- ^ Liebzeit KA, Markowitz JS, Caley CF (February 2001). "New onset diabetes and atypical antipsychotics". European Neuropsychopharmacology 11 (1): 25–32. doi:10.1016/S0924-977X(00)00127-9. PMID 11226809.
- ^ Citrome, L.L. (2004). "The increase in risk of diabetes mellitus from exposure to second-generation antipsychotic agents". Drugs of Today 40 (5): 445–64. doi:10.1358/dot.2004.40.5.850492. PMID 15319799.
- ^ Calabrese, Edward J. (1991). Alcohol interactions with drugs and chemicals. Informa Healthcare. p. 44. ISBN 0873714032. http://books.google.com/?id=FexfBJEYutoC&pg=PA44&lpg=PA44&dq=chlorpromazine+with+ethanol&q=chlorpromazine%20with%20ethanol.
- ^ a b Shin JG, Soukhova N, Flockhart DA (September 1999). "Effect of antipsychotic drugs on human liver cytochrome P-450 (CYP) isoforms in vitro: preferential inhibition of CYP2D6". Drug Metabolism and Disposition 27 (9): 1078–84. PMID 10460810. http://dmd.aspetjournals.org/cgi/pmidlookup?view=long&pmid=10460810.
- ^ a b Daniel WA, Syrek M, Ryłko Z, Kot M (2001). "Effects of phenothiazine neuroleptics on the rate of caffeine demethylation and hydroxylation in the rat liver". Polish Journal of Pharmacology 53 (6): 615–21. PMID 11985335. http://www.if-pan.krakow.pl/pjp/pdf/2001/6_615.pdf.
- ^ Thomas D, Wu K, Kathöfer S et al. (June 2003). "The antipsychotic drug chlorpromazine inhibits HERG potassium channels". British Journal of Pharmacology 139 (3): 567–74. doi:10.1038/sj.bjp.0705283. PMC 1573882. PMID 12788816. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=1573882.
- ^ Razeghinejad, Mohammad Reza; Nowroozzadeh, Mohammad Hosein; Zamani, Mohammad; Amini, Nima (2008). "In vivoobservations of chlorpromazine ocular deposits in a patient on long-term chlorpromazine therapy". Clinical & Experimental Ophthalmology 36 (6): 560–3. doi:10.1111/j.1442-9071.2008.01832.x. PMID 18954320.
- ^ Martin JV, Hague RV, Martin PJ, Cullen DR, Goldberg DM (August 1976). "The association between serum triglycerides and gamma glutamyl transpeptidase activity in diabetes mellitus". Clinical Biochemistry 9 (4): 208–11. doi:10.1016/S0009-9120(76)80059-8. PMID 8220.
- ^ Wiles DH, Orr MW, Kolakowska T (March 1978). "Chlorpromazine levels in plasma and milk of nursing mothers". British Journal of Clinical Pharmacology 5 (3): 272–3. PMC 1429278. PMID 656275. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=1429278.
- ^ Hammond JE, Toseland PA (February 1970). "Placental transfer of chlorpromazine. Case report". Archives of Disease in Childhood 45 (239): 139–40. doi:10.1136/adc.45.239.139. PMC 2020419. PMID 5440181. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2020419.
- ^ a b c d e Yeung PK, Hubbard JW, Korchinski ED, Midha KK (1993). "Pharmacokinetics of chlorpromazine and key metabolites". European Journal of Clinical Pharmacology 45 (6): 563–9. doi:10.1007/BF00315316. PMID 8157044.
- ^ Dahl SG, Strandjord RE (April 1977). "Pharmacokinetics of chlorpromazine after single and chronic dosage". Clinical Pharmacology and Therapeutics 21 (4): 437–48. PMID 849674.
- ^ Gilman, Alfred; Goodman, Louis Sanford; Hardman, Joel G.; Limbird, Lee E., ed (2001). Goodman & Gilman's the pharmacological basis of therapeutics (10th ed.). New York: McGraw-Hill. p. 498. ISBN 978-0-07-135469-1.
- ^ Girault J, Greengard P (2004). "The neurobiology of dopamine signaling". Arch Neurol 61 (5): 641–44. doi:10.1001/archneur.61.5.641. PMID 15148138.
- ^ Peroutka SJ, Synder SH (December 1980). "Relationship of neuroleptic drug effects at brain dopamine, serotonin, alpha-adrenergic, and histamine receptors to clinical potency". The American Journal of Psychiatry 137 (12): 1518–22. PMID 6108081. http://ajp.psychiatryonline.org/cgi/pmidlookup?view=long&pmid=6108081.
- ^ Lidsky TI, Yablonsky-Alter E, Zuck LG, Banerjee SP (August 1997). "Antipsychotic drug effects on glutamatergic activity". Brain Research 764 (1–2): 46–52. doi:10.1016/S0006-8993(97)00423-X. PMID 9295192.
- ^ Falkai, P; Vogeley K (2000 April). "The chances of new atypical substances". biopsychiatry.com. http://www.biopsychiatry.com/antipsychotics.htm. Retrieved 6 July 2010.
- ^ Group, BMJ, ed (March 2009). "4.2.1". British National Formulary (57 ed.). United Kingdom: Royal Pharmaceutical Society of Great Britain. p. 192. ISBN 0260-535X. "Withdrawal of antipsychotic drugs after long-term therapy should always be gradual and closely monitored to avoid the risk of acute withdrawal syndromes or rapid relapse."
- ^ P. Charpentier, U.S. Patent 2,645,640 (1953)
- ^ P. Charpentier, DE 910301 (1951)
- ^ P. Charpentier, P. Gailliot, R. Jacob, J. Gaudechon, P. Buisson, Compt. Rend., 235, 59 (1952)
Bibliography
- Baldessarini, Ross J.; Frank I. Tarazi (2006). "Pharmacotherapy of Psychosis and Mania". In Laurence Brunton, John Lazo, Keith Parker (eds.). Goodman & Gilman's The Pharmacological Basis of Therapeutics (11th ed.). New York: McGraw-Hill. ISBN 978-0071422802.
- Bezchlibnyk-Butler, K. Z. Clinical Handbook of Psychotropic Drugs (German Edition)
- Rote Liste (German Drug Compendium)
- Benkert, O. and H. Hippius. Psychiatrische Pharmakotherapie (German. 6th Edition, 1996)
- Physician's Desktop Reference (2004)
- Heinrich, K. Psychopharmaka in Klinik und Praxis (German, 2nd Edition, 1983)
- Römpp, Chemielexikon (German, 9th Edition)
- NINDS Information Homepage (see External links section)
- Plumb, Dondal C. Plumb's Veterinary Drug Handbook (Blackwell, 5th Edition, 2005)
- "Methods of Execution". Clark County, IN Prosecuting Attorney web page. http://www.clarkprosecutor.org/html/death/methods.htm. Retrieved 2008-08-03.
External links
- Medlineplus article on chlorpromazine
- National Institute of Neurological Disorders and Stroke (NINDS) Tardive Dyskinesia information page
- U.S. National Library of Medicine: Drug Information Portal - Chlorpromazine
Adrenergics Receptor ligands Agonists: 5-FNE • 6-FNE • Amidephrine • Anisodamine • Anisodine • Cirazoline • Dipivefrine • Dopamine • Ephedrine • Epinephrine (Adrenaline) • Etilefrine • Ethylnorepinephrine • Indanidine • Levonordefrin • Metaraminol • Methoxamine • Methyldopa • Midodrine • Naphazoline • Norepinephrine (Noradrenaline) • Octopamine • Oxymetazoline • Phenylephrine • Phenylpropanolamine • Pseudoephedrine • Synephrine • Tetrahydrozoline
Antagonists: Abanoquil • Adimolol • Ajmalicine • Alfuzosin • Amosulalol • Arotinolol • Atiprosin • Benoxathian • Buflomedil • Bunazosin • Carvedilol • CI-926 • Corynanthine • Dapiprazole • DL-017 • Domesticine • Doxazosin • Eugenodilol • Fenspiride • GYKI-12,743 • GYKI-16,084 • Indoramin • Ketanserin • L-765,314 • Labetalol • Mephendioxan • Metazosin • Monatepil • Moxisylyte (Thymoxamine) • Naftopidil • Nantenine • Neldazosin • Nicergoline • Niguldipine • Pelanserin • Phendioxan • Phenoxybenzamine • Phentolamine • Piperoxan • Prazosin • Quinazosin • Ritanserin • RS-97,078 • SGB-1,534 • Silodosin • SL-89.0591 • Spiperone • Talipexole • Tamsulosin • Terazosin • Tibalosin • Tiodazosin • Tipentosin • Tolazoline • Trimazosin • Upidosin • Urapidil • Zolertine
* Note that many TCAs, TeCAs, antipsychotics, ergolines, and some piperazines like buspirone, trazodone, nefazodone, etoperidone, and mepiprazole all antagonize α1-adrenergic receptors as well, which contributes to their side effects such as orthostatic hypotension.Agonists: (R)-3-Nitrobiphenyline • 4-NEMD • 6-FNE • Amitraz • Apraclonidine • Brimonidine • Cannabivarin • Clonidine • Detomidine • Dexmedetomidine • Dihydroergotamine • Dipivefrine • Dopamine • Ephedrine • Ergotamine • Epinephrine (Adrenaline) • Esproquin • Etilefrine • Ethylnorepinephrine • Guanabenz • Guanfacine • Guanoxabenz • Levonordefrin • Lofexidine • Medetomidine • Methyldopa • Mivazerol • Naphazoline • Norepinephrine (Noradrenaline) • Phenylpropanolamine • Piperoxan • Pseudoephedrine • Rilmenidine • Romifidine • Talipexole • Tetrahydrozoline • Tizanidine • Tolonidine • Urapidil • Xylazine • Xylometazoline
Antagonists: 1-PP • Adimolol • Aptazapine • Atipamezole • BRL-44408 • Buflomedil • Cirazoline • Efaroxan • Esmirtazapine • Fenmetozole • Fluparoxan • GYKI-12,743 • GYKI-16,084 • Idazoxan • Mianserin • Mirtazapine • MK-912 • NAN-190 • Olanzapine • Phentolamine • Phenoxybenzamine • Piperoxan • Piribedil • Rauwolscine • Rotigotine • SB-269,970 • Setiptiline • Spiroxatrine • Sunepitron • Tolazoline • Yohimbine
* Note that many atypical antipsychotics and azapirones like buspirone and gepirone (via metabolite 1-PP) antagonize α2-adrenergic receptors as well.βAgonists: 2-FNE • 5-FNE • Amibegron • Arbutamine • Arformoterol • Arotinolol • BAAM • Bambuterol • Befunolol • Bitolterol • Broxaterol • Buphenine • Carbuterol • Cimaterol • Clenbuterol • Denopamine • Deterenol • Dipivefrine • Dobutamine • Dopamine • Dopexamine • Ephedrine • Epinephrine (Adrenaline) • Etafedrine • Etilefrine • Ethylnorepinephrine • Fenoterol • Formoterol • Hexoprenaline • Higenamine • Indacaterol • Isoetarine • Isoprenaline (Isoproterenol) • Isoxsuprine • Labetalol • Levonordefrin • Levosalbutamol • Mabuterol • Methoxyphenamine • Methyldopa • Norepinephrine (Noradrenaline) • Orciprenaline • Oxyfedrine • Phenylpropanolamine • Pirbuterol • Prenalterol • Ractopamine • Procaterol • Pseudoephedrine • Reproterol • Rimiterol • Ritodrine • Salbutamol (Albuterol) • Salmeterol • Solabegron • Terbutaline • Tretoquinol • Tulobuterol • Xamoterol • Zilpaterol • Zinterol
Antagonists: Acebutolol • Adaprolol • Adimolol • Afurolol • Alprenolol • Alprenoxime • Amosulalol • Ancarolol • Arnolol • Arotinolol • Atenolol • Befunolol • Betaxolol • Bevantolol • Bisoprolol • Bopindolol • Bormetolol • Bornaprolol • Brefonalol • Bucindolol • Bucumolol • Bufetolol • Buftiralol • Bufuralol • Bunitrolol • Bunolol • Bupranolol • Burocrolol • Butaxamine • Butidrine • Butofilolol • Capsinolol • Carazolol • Carpindolol • Carteolol • Carvedilol • Celiprolol • Cetamolol • Cicloprolol • Cinamolol • Cloranolol • Cyanopindolol • Dalbraminol • Dexpropranolol • Diacetolol • Dichloroisoprenaline • Dihydroalprenolol • Dilevalol • Diprafenone • Draquinolol • Dropranolol • Ecastolol • Epanolol • Ericolol • Ersentilide • Esatenolol • Esmolol • Esprolol • Eugenodilol • Exaprolol • Falintolol • Flestolol • Flusoxolol • Hydroxycarteolol • Hydroxytertatolol • ICI-118,551 • Idropranolol • Indenolol • Indopanolol • Iodocyanopindolol • Iprocrolol • Isoxaprolol • Isamoltane • Labetalol • Landiolol • Levobetaxolol • Levobunolol • Levocicloprolol • Levomoprolol • Medroxalol • Mepindolol • Metalol • Metipranolol • Metoprolol • Moprolol • Nadolol • Nadoxolol • Nafetolol • Nebivolol • Neraminol • Nifenalol • Nipradilol • Oberadilol • Oxprenolol • Pacrinolol • Pafenolol • Pamatolol • Pargolol • Parodilol • Penbutolol • Penirolol • PhQA-33 • Pindolol • Pirepolol • Practolol • Primidolol • Procinolol • Pronethalol • Propafenone • Propranolol • Ridazolol • Ronactolol • Soquinolol • Sotalol • Spirendolol • SR 59230A • Sulfinalol • TA-2005 • Talinolol • Tazolol • Teoprolol • Tertatolol • Terthianolol • Tienoxolol • Tilisolol • Timolol • Tiprenolol • Tolamolol • Toliprolol • Tribendilol • Trigevolol • Xibenolol • XipranololReuptake inhibitors Selective norepinephrine reuptake inhibitors: Amedalin • Atomoxetine (Tomoxetine) • Ciclazindol • Daledalin • Esreboxetine • Lortalamine • Mazindol • Nisoxetine • Reboxetine • Talopram • Talsupram • Tandamine • Viloxazine; Norepinephrine-dopamine reuptake inhibitors: Amineptine • Bupropion (Amfebutamone) • Fencamine • Fencamfamine • Lefetamine • Levophacetoperane • LR-5182 • Manifaxine • Methylphenidate • Nomifensine • O-2172 • Radafaxine; Serotonin-norepinephrine reuptake inhibitors: Bicifadine • Desvenlafaxine • Duloxetine • Eclanamine • Levomilnacipran • Milnacipran • Sibutramine • Venlafaxine; Serotonin-norepinephrine-dopamine reuptake inhibitors: Brasofensine • Diclofensine • DOV-102,677 • DOV-21,947 • DOV-216,303 • JNJ-7925476 • JZ-IV-10 • Methylnaphthidate • Naphyrone • NS-2359 • PRC200-SS • SEP-225,289 • SEP-227,162 • Tesofensine; Tricyclic antidepressants: Amitriptyline • Butriptyline • Cianopramine • Clomipramine • Desipramine • Dosulepin • Doxepin • Imipramine • Lofepramine • melitracen • Nortriptyline • Protriptyline • Trimipramine; Tetracyclic antidepressants: Amoxapine • Maprotiline • Mianserin • Oxaprotiline • Setiptiline; Others: Cocaine • CP-39,332 • EXP-561 • Fezolamine • Ginkgo biloba • Indeloxazine • Nefazodone • Nefopam • Pridefrine • Tapentadol • Teniloxazine • Tramadol • ZiprasidoneReleasing agents Morpholines: Fenbutrazate • Morazone • Phendimetrazine • Phenmetrazine; Oxazolines: 4-Methylaminorex • Aminorex • Clominorex • Cyclazodone • Fenozolone • Fluminorex • Pemoline • Thozalinone; Phenethylamines (also amphetamines, cathinones, phentermines, etc): 2-OH-PEA • 4-CAB • 4-FA • 4-FMA • 4-MA • 4-MMA • Alfetamine • Amfecloral • Amfepentorex • Amfepramone • Amphetamine (Dextroamphetamine, Levoamphetamine) • Amphetaminil • β-Me-PEA • BDB • Benzphetamine • BOH • Buphedrone • Butylone • Cathine • Cathinone • Clobenzorex • Clortermine • D-Deprenyl • Dimethylamphetamine • Dimethylcathinone (Dimethylpropion, metamfepramone) • DMA • DMMA • EBDB • Ephedrine • Ethcathinone • Ethylamphetamine • Ethylone • Famprofazone • Fenethylline • Fenproporex • Flephedrone • Fludorex • Furfenorex • Hordenine • IAP • IMP • L-Deprenyl (Selegiline) • Lisdexamfetamine • Lophophine • MBDB • MDA (Tenamfetamine) • MDEA • MDMA • MDMPEA • MDOH • MDPEA • Mefenorex • Mephedrone • Mephentermine • Methamphetamine (Dextromethamphetamine, Levomethamphetamine) • Methcathinone • Methedrone • Methylone • NAP • Ortetamine • Paredrine • pBA • pCA • Pentorex (Phenpentermine) • Phenethylamine • Pholedrine • Phenpromethamine • Phentermine • Phenylpropanolamine • pIA • Prenylamine • Propylamphetamine • Pseudoephedrine • Tiflorex • Tyramine • Xylopropamine • Zylofuramine; Piperazines: 2C-B-BZP • BZP • MBZP • mCPP • MDBZP • MeOPP • pFPP; Others: 2-Amino-1,2-dihydronaphthalene • 2-Aminoindane • 2-Aminotetralin • 2-Benzylpiperidine • 4-Benzylpiperidine • 5-IAI • Clofenciclan • Cyclopentamine • Cypenamine • Cyprodenate • Feprosidnine • Gilutensin • Heptaminol • Hexacyclonate • Indanorex • Isometheptene • Methylhexanamine • Octodrine • Phthalimidopropiophenone • Propylhexedrine (Levopropylhexedrine) • TuaminoheptaneEnzyme inhibitors 3,4-DihydroxystyreneDBHCGS-19281A • SKF-64139 • SKF-7698Nonselective: Benmoxin • Caroxazone • Echinopsidine • Furazolidone • Hydralazine • Indantadol • Iproclozide • Iproniazid • Isocarboxazid • Isoniazid • Linezolid • Mebanazine • Metfendrazine • Nialamide • Octamoxin • Paraxazone • Phenelzine • Pheniprazine • Phenoxypropazine • Pivalylbenzhydrazine • Procarbazine • Safrazine • Tranylcypromine; MAO-A selective: Amiflamine • Bazinaprine • Befloxatone • Befol • Brofaromine • Cimoxatone • Clorgiline • Esuprone • Harmala alkaloids (Harmine, Harmaline, Tetrahydroharmine, Harman, Norharman, etc) • Methylene Blue • Metralindole • Minaprine • Moclobemide • Pirlindole • Sercloremine • Tetrindole • Toloxatone • Tyrima; MAO-B selective: D-Deprenyl • Selegiline (L-Deprenyl) • Ladostigil • Lazabemide • Milacemide • Mofegiline • Pargyline • Rasagiline • Safinamide
* Note that MAO-B inhibitors also influence norepinephrine/epinephrine levels since they inhibit the breakdown of their precursor dopamine.COMTOthers Ferrous Iron (Fe2+) • S-Adenosyl-L-Methionine • Vitamin B3 (Niacin, Nicotinamide → NADPH) • Vitamin B6 (Pyridoxine, Pyridoxamine, Pyridoxal → Pyridoxal Phosphate) • Vitamin B9 (Folic acid → Tetrahydrofolic acid) • Vitamin C (Ascorbic acid) • Zinc (Zn2+)OthersActivity enhancers: BPAP • PPAP; Release blockers: Bethanidine • Bretylium • Guanadrel • Guanazodine • Guanclofine • Guanethidine • Guanoxan; Toxins: Oxidopamine (6-Hydroxydopamine)List of adrenergic drugsCholinergics Receptor ligands Agonists: 77-LH-28-1 • AC-42 • AC-260,584 • Aceclidine • Acetylcholine • AF30 • AF150(S) • AF267B • AFDX-384 • Alvameline • AQRA-741 • Arecoline • Bethanechol • Butyrylcholine • Carbachol • CDD-0034 • CDD-0078 • CDD-0097 • CDD-0098 • CDD-0102 • Cevimeline • cis-Dioxolane • Ethoxysebacylcholine • LY-593,039 • L-689,660 • LY-2,033,298 • McNA343 • Methacholine • Milameline • Muscarine • NGX-267 • Ocvimeline • Oxotremorine • PD-151,832 • Pilocarpine • RS86 • Sabcomeline • SDZ 210-086 • Sebacylcholine • Suberylcholine • Talsaclidine • Tazomeline • Thiopilocarpine • Vedaclidine • VU-0029767 • VU-0090157 • VU-0152099 • VU-0152100 • VU-0238429 • WAY-132,983 • Xanomeline • YM-796
Antagonists: 3-Quinuclidinyl Benzilate • 4-DAMP • Aclidinium Bromide • Anisodamine • Anisodine • Atropine • Atropine Methonitrate • Benactyzine • Benzatropine (Benztropine) • Benzydamine • BIBN 99 • Biperiden • Bornaprine • CAR-226,086 • CAR-301,060 • CAR-302,196 • CAR-302,282 • CAR-302,368 • CAR-302,537 • CAR-302,668 • CS-27349 • Cyclobenzaprine • Cyclopentolate • Darifenacin • DAU-5884 • Dimethindene • Dexetimide • DIBD • Dicyclomine (Dicycloverine) • Ditran • EA-3167 • EA-3443 • EA-3580 • EA-3834 • Elemicin • Etanautine • Etybenzatropine (Ethylbenztropine) • Flavoxate • Himbacine • HL-031,120 • Ipratropium bromide • J-104,129 • Hyoscyamine • Mamba Toxin 3 • Mamba Toxin 7 • Mazaticol • Mebeverine • Methoctramine • Metixene • Myristicin • N-Ethyl-3-Piperidyl Benzilate • N-Methyl-3-Piperidyl Benzilate • Orphenadrine • Otenzepad • Oxybutynin • PBID • PD-102,807 • PD-0298029 • Phenglutarimide • Phenyltoloxamine • Pirenzepine • Piroheptine • Procyclidine • Profenamine • RU-47,213 • SCH-57,790 • SCH-72,788 • SCH-217,443 • Scopolamine (Hyoscine) • Solifenacin • Telenzepine • Tiotropium bromide • Tolterodine • Trihexyphenidyl • Tripitamine • Tropatepine • Tropicamide • WIN-2299 • Xanomeline • Zamifenacin; Others: 1st Generation Antihistamines (Brompheniramine, chlorphenamine, cyproheptadine, dimenhydrinate, diphenhydramine, doxylamine, mepyramine/pyrilamine, phenindamine, pheniramine, tripelennamine, triprolidine, etc) • Tricyclic Antidepressants (Amitriptyline, doxepin, trimipramine, etc) • Tetracyclic Antidepressants (Amoxapine, maprotiline, etc) • Typical Antipsychotics (Chlorpromazine, thioridazine, etc) • Atypical Antipsychotics (Clozapine, olanzapine, quetiapine, etc)Agonists: 5-HIAA • A-84,543 • A-366,833 • A-582,941 • A-867,744 • ABT-202 • ABT-418 • ABT-560 • ABT-894 • Acetylcholine • Altinicline • Anabasine • Anatoxin-a • AR-R17779 • Butyrylcholine • Carbachol • Cotinine • Cytisine • Decamethonium • Desformylflustrabromine • Dianicline • Dimethylphenylpiperazinium • Epibatidine • Epiboxidine • Ethanol • Ethoxysebacylcholine • EVP-4473 • EVP-6124 • Galantamine • GTS-21 • Ispronicline • Lobeline • MEM-63,908 (RG-3487) • Nicotine • NS-1738 • PHA-543,613 • PHA-709,829 • PNU-120,596 • PNU-282,987 • Pozanicline • Rivanicline • Sazetidine A • Sebacylcholine • SIB-1508Y • SIB-1553A • SSR-180,711 • Suberylcholine • TC-1698 • TC-1734 • TC-1827 • TC-2216 • TC-5214 • TC-5619 • TC-6683 • Tebanicline • Tropisetron • UB-165 • Varenicline • WAY-317,538 • XY-4083
Antagonists: 18-Methoxycoronaridine • α-Bungarotoxin • α-Conotoxin • Alcuronium • Amantadine • Anatruxonium • Atracurium • Bupropion (Amfebutamone) • Chandonium • Chlorisondamine • Cisatracurium • Coclaurine • Coronaridine • Dacuronium • Decamethonium • Dextromethorphan • Dextropropoxyphene • Dextrorphan • Diadonium • DHβE • Dimethyltubocurarine (Metocurine) • Dipyrandium • Dizocilpine (MK-801) • Doxacurium • Duador • Esketamine • Fazadinium • Gallamine • Hexafluronium • Hexamethonium (Benzohexonium) • Ibogaine • Isoflurane • Ketamine • Kynurenic acid • Laudexium (Laudolissin) • Levacetylmethadol • Malouetine • Mecamylamine • Memantine • Methadone • Methorphan (Racemethorphan) • Methyllycaconitine • Metocurine • Mivacurium • Morphanol (Racemorphanol) • Neramexane • Nitrous Oxide • Pancuronium • Pempidine • Pentamine • Pentolinium • Phencyclidine • Pipecuronium • Radafaxine • Rapacuronium • Rocuronium • Surugatoxin • Suxamethonium (Succinylcholine) • Thiocolchicoside • Toxiferine • Trimethaphan • Tropeinium • Tubocurarine • Vecuronium • XenonReuptake inhibitors PlasmalemmalCHT InhibitorsHemicholinium-3 (Hemicholine; HC3) • TriethylcholineVAChT InhibitorsEnzyme inhibitors ChAT inhibitors1-(-Benzoylethyl)pyridinium • 2-(α-Naphthoyl)ethyltrimethylammonium • 3-Chloro-4-stillbazole • 4-(1-Naphthylvinyl)pyridine • Acetylseco hemicholinium-3 • Acryloylcholine • AF64A • B115 • BETA • CM-54,903 • CatabolismAChE inhibitorsReversible: Carbamates: Aldicarb • Bendiocarb • Bufencarb • Carbaryl • Carbendazim • Carbetamide • Carbofuran • Chlorbufam • Chloropropham • Ethienocarb • Ethiofencarb • Fenobucarb • Fenoxycarb • Formetanate • Furadan • Ladostigil • Methiocarb • Methomyl • Miotine • Oxamyl • Phenmedipham • Pinmicarb • Pirimicarb • Propamocarb • Propham • Propoxur; Stigmines: Ganstigmine • Neostigmine • Phenserine • Physostigmine • Pyridostigmine • Rivastigmine; Others: Acotiamide • Ambenonium • Donepezil • Edrophonium • Galantamine • Huperzine A • Minaprine • Tacrine • Zanapezil
Irreversible: Organophosphates: Acephate • Azinphos-methyl • Bensulide • Cadusafos • Chlorethoxyfos • Chlorfenvinphos • Chlorpyrifos • Chlorpyrifos-Methyl • Coumaphos • Cyclosarin (GF) • Demeton • Demeton-S-Methyl • Diazinon • Dichlorvos • Dicrotophos • Diisopropyl fluorophosphate (Guthion) • Diisopropylphosphate • Dimethoate • Dioxathion • Disulfoton • EA-3148 • Echothiophate • Ethion • Ethoprop • Fenamiphos • Fenitrothion • Fenthion • Fosthiazate • GV • Isofluorophate • Isoxathion • Malaoxon • Malathion • Methamidophos • Methidathion • Metrifonate • Mevinphos • Monocrotophos • Naled • Novichok agent • Omethoate • Oxydemeton-Methyl • Paraoxon • Parathion • Parathion-Methyl • Phorate • Phosalone • Phosmet • Phostebupirim • Phoxim • Pirimiphos-Methyl • Sarin (GB) • Soman (GD) • Tabun (GA) • Temefos • Terbufos • Tetrachlorvinphos • Tribufos • Trichlorfon • VE • VG • VM • VR • VX; Others: Demecarium • Onchidal (Onchidella binneyi)BChE inhibitorsCymserine * Many of the acetylcholinesterase inhibitors listed above act as butyrylcholinesterase inhibitors.Others Choline (Lecithin) • Citicoline • Cyprodenate • Dimethylethanolamine (DMAE, deanol) • Glycerophosphocholine • Meclofenoxate (Centrophenoxine) • Phosphatidylcholine • Phosphatidylethanolamine • Phosphorylcholine • PirisudanolOthersAcetylcholine releasing agents: α-Latrotoxin • β-Bungarotoxin; Acetylcholine release inhibitors: Botulinum toxin (Botox); Acetylcholinesterase reactivators: Asoxime • Obidoxime • PralidoximeDopaminergics Receptor ligands AgonistsAdamantanes: Amantadine • Memantine • Rimantadine; Aminotetralins: 7-OH-DPAT • 8-OH-PBZI • Rotigotine • UH-232; Benzazepines: 6-Br-APB • Fenoldopam • SKF-38,393 • SKF-77,434 • SKF-81,297 • SKF-82,958 • SKF-83,959; Ergolines: Bromocriptine • Cabergoline • Dihydroergocryptine • Lisuride • LSD • Pergolide; Dihydrexidine derivatives: 2-OH-NPA • A-86,929 • Ciladopa • Dihydrexidine • Dinapsoline • Dinoxyline • Doxanthrine; Others: A-68,930 • A-77,636 • A-412,997 • ABT-670 • ABT-724 • Aplindore • Apomorphine • Aripiprazole • Bifeprunox • BP-897 • CY-208,243 • Dizocilpine • Etilevodopa • Flibanserin • Ketamine • Melevodopa • Modafinil • Pardoprunox • Phencyclidine • PD-128,907 • PD-168,077 • PF-219,061 • Piribedil • Pramipexole • Propylnorapomorphine • Pukateine • Quinagolide • Quinelorane • Quinpirole • RDS-127 • Ro10-5824 • Ropinirole • Rotigotine • Roxindole • Salvinorin A • SKF-89,145 • Sumanirole • Terguride • Umespirone • WAY-100,635AntagonistsTypical antipsychotics: Acepromazine • Azaperone • Benperidol • Bromperidol • Clopenthixol • Chlorpromazine • Chlorprothixene • Droperidol • Flupentixol • Fluphenazine • Fluspirilene • Haloperidol • Loxapine • Mesoridazine • Methotrimeprazine • Nemonapride • Penfluridol • Perazine • Periciazine • Perphenazine • Pimozide • Prochlorperazine • Promazine • Sulforidazine • Sulpiride • Sultopride • Thioridazine • Thiothixene • Trifluoperazine • Triflupromazine • Trifluperidol • Zuclopenthixol; Atypical antipsychotics: Amisulpride • Asenapine • Blonanserin • Cariprazine • Carpipramine • Clocapramine • Clozapine • Gevotroline • Iloperidone • Lurasidone • Melperone • Molindone • Mosapramine • Olanzapine • Paliperidone • Perospirone • Piquindone • Quetiapine • Remoxipride • Risperidone • Sertindole • Tiospirone • Ziprasidone • Zotepine; Antiemetics: AS-8112 • Alizapride • Bromopride • Clebopride • Domperidone • Metoclopramide • Thiethylperazine; Others: Amoxapine • Buspirone • Butaclamol • Ecopipam • EEDQ • Eticlopride • Fananserin • L-745,870 • Nafadotride • Nuciferine • PNU-99,194 • Raclopride • Sarizotan • SB-277,011-A • SCH-23,390 • SKF-83,959 • Sonepiprazole • Spiperone • Spiroxatrine • Stepholidine • Tetrahydropalmatine • Tiapride • UH-232 • YohimbineReuptake inhibitors PlasmalemmalDAT inhibitorsPiperazines: DBL-583 • GBR-12,935 • Nefazodone • Vanoxerine; Piperidines: BTCP • Desoxypipradrol • Dextromethylphenidate • Difemetorex • Ethylphenidate • Methylnaphthidate • Methylphenidate • Phencyclidine • Pipradrol; Pyrrolidines: Diphenylprolinol • Methylenedioxypyrovalerone (MDPV) • Naphyrone • Prolintane • Pyrovalerone; Tropanes: β-CPPIT • Altropane • Brasofensine • CFT • Cocaine • Dichloropane • Difluoropine • FE-β-CPPIT • FP-β-CPPIT • Ioflupane (123I) • Iometopane • RTI-112 • RTI-113 • RTI-121 • RTI-126 • RTI-150 • RTI-177 • RTI-229 • RTI-336 • Tenocyclidine • Tesofensine • Troparil • Tropoxane • WF-11 • WF-23 • WF-31 • WF-33; Others: Adrafinil • Armodafinil • Amfonelic acid • Amineptine • Benzatropine (Benztropine) • Bromantane • BTQ • BTS-74,398 • Bupropion (Amfebutamone) • Ciclazindol • Diclofensine • Dimethocaine • Diphenylpyraline • Dizocilpine • DOV-102,677 • DOV-21,947 • DOV-216,303 • Etybenzatropine (Ethylbenztropine) • EXP-561 • Fencamine • Fencamfamine • Fezolamine • GYKI-52,895 • Indatraline • Ketamine • Lefetamine • Levophacetoperane • LR-5182 • Manifaxine • Mazindol • Medifoxamine • Mesocarb • Modafinil • Nefopam • Nomifensine • NS-2359 • O-2172 • Pridefrine • Propylamphetamine • Radafaxine • SEP-225,289 • SEP-227,162 • Sertraline • Sibutramine • Tametraline • TripelennamineVMAT inhibitorsReleasing agents Morpholines: Fenbutrazate • Morazone • Phendimetrazine • Phenmetrazine; Oxazolines: 4-Methylaminorex (4-MAR, 4-MAX) • Aminorex • Clominorex • Cyclazodone • Fenozolone • Fluminorex • Pemoline • Thozalinone; Phenethylamines (also amphetamines, cathinones, phentermines, etc): 2-Hydroxyphenethylamine (2-OH-PEA) • 4-CAB • 4-Methylamphetamine (4-MA) • 4-Methylmethamphetamine (4-MMA) • Alfetamine • Amfecloral • Amfepentorex • Amfepramone • Amphetamine (Dextroamphetamine, Levoamphetamine) • Amphetaminil • β-Methylphenethylamine (β-Me-PEA) • Benzodioxolylbutanamine (BDB) • Benzodioxolylhydroxybutanamine (BOH) • Benzphetamine • Buphedrone • Butylone • Cathine • Cathinone • Clobenzorex • Clortermine • D-Deprenyl • Dimethoxyamphetamine (DMA) • Dimethoxymethamphetamine (DMMA) • Dimethylamphetamine • Dimethylcathinone (Dimethylpropion, metamfepramone) • Ethcathinone (Ethylpropion) • Ethylamphetamine • Ethylbenzodioxolylbutanamine (EBDB) • Ethylone • Famprofazone • Fenethylline • Fenproporex • Flephedrone • Fludorex • Furfenorex • Hordenine • Lophophine (Homomyristicylamine) • Mefenorex • Mephedrone • Methamphetamine (Desoxyephedrine, Methedrine; Dextromethamphetamine, Levomethamphetamine) • Methcathinone (Methylpropion) • Methedrone • Methoxymethylenedioxyamphetamine (MMDA) • Methoxymethylenedioxymethamphetamine (MMDMA) • Methylbenzodioxolylbutanamine (MBDB) • Methylenedioxyamphetamine (MDA, tenamfetamine) • Methylenedioxyethylamphetamine (MDEA) • Methylenedioxyhydroxyamphetamine (MDOH) • Methylenedioxymethamphetamine (MDMA) • Methylenedioxymethylphenethylamine (MDMPEA, homarylamine) • Methylenedioxyphenethylamine (MDPEA, homopiperonylamine) • Methylone • Ortetamine • Parabromoamphetamine (PBA) • Parachloroamphetamine (PCA) • Parafluoroamphetamine (PFA) • Parafluoromethamphetamine (PFMA) • Parahydroxyamphetamine (PHA) • Paraiodoamphetamine (PIA) • Paredrine (Norpholedrine, Oxamphetamine) • Phenethylamine (PEA) • Pholedrine • Phenpromethamine • Prenylamine • Propylamphetamine • Tiflorex (Flutiorex) • Tyramine (TRA) • Xylopropamine • Zylofuramine; Piperazines: 2,5-Dimethoxy-4-bromobenzylpiperazine (2C-B-BZP) • Benzylpiperazine (BZP) • Methoxyphenylpiperazine (MeOPP, paraperazine) • Methylbenzylpiperazine (MBZP) • Methylenedioxybenzylpiperazine (MDBZP, piperonylpiperazine); Others: 2-Amino-1,2-dihydronaphthalene (2-ADN) • 2-Aminoindane (2-AI) • 2-Aminotetralin (2-AT) • 4-Benzylpiperidine (4-BP) • 5-IAI • Clofenciclan • Cyclopentamine • Cypenamine • Cyprodenate • Feprosidnine • Gilutensin • Heptaminol • Hexacyclonate • Indanylaminopropane (IAP) • Indanorex • Isometheptene • Methylhexanamine • Naphthylaminopropane (NAP) • Octodrine • Phthalimidopropiophenone • Propylhexedrine (Levopropylhexedrine) • Tuaminoheptane (Tuamine)Enzyme inhibitors PAH inhibitors3,4-DihydroxystyreneTH inhibitorsNonselective: Benmoxin • Caroxazone • Echinopsidine • Furazolidone • Hydralazine • Indantadol • Iproclozide • Iproniazid • Isocarboxazid • Isoniazid • Linezolid • Mebanazine • Metfendrazine • Nialamide • Octamoxin • Paraxazone • Phenelzine • Pheniprazine • Phenoxypropazine • Pivalylbenzhydrazine • Procarbazine • Safrazine • Tranylcypromine; MAO-A selective: Amiflamine • Bazinaprine • Befloxatone • Befol • Brofaromine • Cimoxatone • Clorgiline • Esuprone • Harmala alkaloids • Methylene Blue • Metralindole • Minaprine • Moclobemide • Pirlindole • Sercloremine • Tetrindole • Toloxatone • Tyrima; MAO-B selective: D-Deprenyl • L-Deprenyl (Selegiline) • Ladostigil • Lazabemide • Milacemide • Pargyline • Rasagiline • SafinamideDBH inhibitorsOthers L-Phenylalanine → L-Tyrosine → L-DOPA (Levodopa)Ferrous iron (Fe2+) • Tetrahydrobiopterin • Vitamin B3 (Niacin, Nicotinamide → NADPH) • Vitamin B6 (Pyridoxine, Pyridoxamine, Pyridoxal → Pyridoxal phosphate) • Vitamin B9 (Folic acid → Tetrahydrofolic acid) • Vitamin C (Ascorbic acid) • Zinc (Zn2+)OthersActivity enhancers: Benzofuranylpropylaminopentane (BPAP) • Phenylpropylaminopentane (PPAP); Toxins: Oxidopamine (6-Hydroxydopamine)List of dopaminergic drugsHistaminergics Receptor
ligandsAgonists: 2-Pyridylethylamine • Betahistine • Histamine • HTMT • UR-AK49
Antagonists: 1st generation: 4-Methyldiphenhydramine • Alimemazine • Antazoline • Azatadine • Bamipine • Benzatropine (Benztropine) • Bepotastine • Bromazine • Brompheniramine • Buclizine • Captodiame • Carbinoxamine • Chlorcyclizine • Chloropyramine • Chlorothen • Chlorphenamine • Chlorphenoxamine • Cinnarizine • Clemastine • Clobenzepam • Clocinizine • Cyclizine • Cyproheptadine • Dacemazine • Deptropine • Dexbrompheniramine • Dexchlorpheniramine • Dimenhydrinate • Dimetindene • Diphenhydramine • Diphenylpyraline • Doxylamine • Embramine • Etybenzatropine (Ethylbenztropine) • Etymemazine • Histapyrrodine • Homochlorcyclizine • Hydroxyethylpromethazine • Hydroxyzine • Isopromethazine • Isothipendyl • Meclozine • Mepyramine (Pyrilamine) • Mequitazine • Methafurylene • Methapyrilene • Methdilazine • Moxastine • Niaprazine • Orphenadrine • Oxatomide • Oxomemazine • Phenindamine • Pheniramine • Phenyltoloxamine • Pimethixene • Piperoxan • Promethazine • Propiomazine • Pyrrobutamine • Talastine • Thenalidine • Thenyldiamine • Thiazinamium • Thonzylamine • Tolpropamine • Tripelennamine • Triprolidine; 2nd generation: Acrivastine • Astemizole • Azelastine • Cetirizine • Clemizole • Clobenztropine • Ebastine • Emedastine • Epinastine • Ketotifen • Latrepirdine • Levocabastine • Loratadine • Mebhydrolin • Mizolastine • Olopatadine • Rupatadine • Setastine • Terfenadine; "3rd generation": Desloratadine • Fexofenadine • Levocetirizine; Miscellaneous: Tricyclic antidepressants (Amitriptyline, Doxepin, Trimipramine, etc) • Tetracyclic antidepressants (Mianserin, Mirtazapine, etc) • Serotonin antagonist and reuptake inhibitors (Trazodone, Nefazodone) • Typical antipsychotics (Chlorpromazine, Thioridazine, etc) • Atypical antipsychotics (Clozapine, Olanzapine, Quetiapine, etc)Agonists: Amthamine • Betazole • Dimaprit • Histamine • HTMT • Impromidine • UR-AK49
Antagonists: Burimamide • Cimetidine • Ebrotidine • Famotidine • Lafutidine • Lavoltidine/Loxtidine • Lupitidine • Metiamide • Niperotidine • Nizatidine • Oxmetidine • Ranitidine • RoxatidineAgonists: α-Methylhistamine • Cipralisant • Histamine • Imetit • Immepip • Immethridine • Methimepip • Proxyfan
Antagonists: A-349,821 • A-423,579 • ABT-239 • Betahistine • Burimamide • Ciproxifan • Clobenpropit • Conessine • GSK-189,254 • Impentamine • Iodophenpropit • JNJ-5,207,852 • MK-0249 • NNC-38-1,049 • PF-03654746 • Pitolisant • SCH-79,687 • Thioperamide • VUF-5,681Agonists: 4-Methylhistamine • Histamine • VUF-8,430
Antagonists: JNJ-7,777,120 • Thioperamide • VUF-6,002Reuptake
inhibitorsVMAT inhibitorsEnzyme
inhibitorsHDC inhibitorsCatechin • Meciadanol • Naringenin • TritoqualineHNMT inhibitorsDAO inhibitorsAminoguanidineOthers L-HistidineSerotonergics 5-HT1 receptor ligands Agonists: Azapirones: Alnespirone • Binospirone • Buspirone • Enilospirone • Eptapirone • Gepirone • Ipsapirone • Perospirone • Revospirone • Tandospirone • Tiospirone • Umespirone • Zalospirone; Antidepressants: Etoperidone • Nefazodone • Trazodone • Vortioxetine; Antipsychotics: Aripiprazole • Asenapine • Clozapine • Quetiapine • Ziprasidone; Ergolines: Dihydroergotamine • Ergotamine • Lisuride • Methysergide • LSD; Tryptamines: 5-CT • 5-MeO-DMT • 5-MT • Bufotenin • DMT • Indorenate • Psilocin • Psilocybin; Others: 8-OH-DPAT • Adatanserin • Befiradol • BMY-14802 • Cannabidiol • Dimemebfe • Ebalzotan • Eltoprazine • F-11,461 • F-12,826 • F-13,714 • F-14,679 • F-15,063 • F-15,599 • Flesinoxan • Flibanserin • Lesopitron • LY-293,284 • LY-301,317 • MKC-242 • NBUMP • Osemozotan • Oxaflozane • Pardoprunox • Piclozotan • Rauwolscine • Repinotan • Roxindole • RU-24,969 • S 14,506 • S-14,671 • S-15,535 • Sarizotan • SSR-181,507 • Sunepitron • U-92,016-A • Urapidil • Vilazodone • Xaliproden • Yohimbine
Antagonists: Antipsychotics: Iloperidone • Risperidone • Sertindole; Beta blockers: Alprenolol • Cyanopindolol • Iodocyanopindolol • Oxprenolol • Pindobind • Pindolol • Propranolol • Tertatolol; Others: AV965 • BMY-7,378 • CSP-2503 • Dotarizine • Flopropione • GR-46611 • Isamoltane • Lecozotan • Mefway • Metitepine/Methiothepin • MPPF • NAN-190 • PRX-00023 • Robalzotan • S-15535 • SB-649,915 • SDZ 216-525 • Spiperone • Spiramide • Spiroxatrine • UH-301 • WAY-100,135 • WAY-100,635 • XylamidineAgonists: Lysergamides: Dihydroergotamine • Ergotamine • Methysergide; Piperazines: Eltoprazine • TFMPP; Triptans: Avitriptan • Eletriptan • Sumatriptan • Zolmitriptan; Tryptamines: 5-CT • 5-MT; Others: CGS-12066A • CP-93,129 • CP-94,253 • CP-135,807 • RU-24,969
Antagonists: Lysergamides: Metergoline; Others: AR-A000002 • Elzasonan • GR-127,935 • Isamoltane • Metitepine/Methiothepin • SB-216,641 • SB-224,289 • SB-236,057 • YohimbineAgonists: Lysergamides: Dihydroergotamine • Methysergide; Triptans: Almotriptan • Avitriptan • Eletriptan • Frovatriptan • Naratriptan • Rizatriptan • Sumatriptan • Zolmitriptan; Tryptamines: 5-CT • 5-Ethyl-DMT • 5-MT • 5-(Nonyloxy)tryptamine; Others: CP-135,807 • CP-286,601 • GR-46611 • L-694,247 • L-772,405 • PNU-109,291 • PNU-142,633
Antagonists: Lysergamides: Metergoline; Others: Alniditan • BRL-15,572 • Elzasonan • GR-127,935 • Ketanserin • LY-310,762 • LY-367,642 • LY-456,219 • LY-456,220 • Metitepine/Methiothepin • Ritanserin • Yohimbine • ZiprasidoneAgonists: Lysergamides: Methysergide; Triptans: Eletriptan; Tryptamines: BRL-54443 • Tryptamine
Antagonists: Metitepine/MethiothepinAgonists: Triptans: Eletriptan • Naratriptan • Sumatriptan; Tryptamines: 5-MT; Others: BRL-54443 • Lasmiditan • LY-334,370
Antagonists: Metitepine/Methiothepin5-HT2 receptor ligands Agonists: Lysergamides: ALD-52 • Ergometrine • Lisuride • LA-SS-Az • LSD • LSD-Pip • Lysergic acid 2-butyl amide • Lysergic acid 3-pentyl amide • Methysergide; Phenethylamines: 25I-NBF • 25I-NBMD • 25I-NBOH • 25I-NBOMe • 2C-B • 2C-B-FLY • 2CB-Ind • 2C-C-NBOMe • 2C-E • 2C-I • 2C-TFM-NBOMe • 2C-T-2 • 2C-T-7 • 2C-T-21 • 2CBCB-NBOMe • 2CBFly-NBOMe • Bromo-DragonFLY • DOB • DOC • DOI • DOM • MDA • MDMA • Mescaline • TCB-2 • TFMFly; Piperazines: BZP • Quipazine • TFMPP; Tryptamines: 5-CT • 5-MeO-α-ET • 5-MeO-α-MT • 5-MeO-DET • 5-MeO-DiPT • 5-MeO-DMT • 5-MeO-DPT • 5-MT • α-ET • α-Methyl-5-HT • α-MT • Bufotenin • DET • DiPT • DMT • DPT • Psilocin • Psilocybin; Others: AL-34662 • AL-37350A • Dimemebfe • Medifoxamine • Oxaflozane • PNU-22394 • RH-34
Antagonists: Atypical antipsychotics: Amperozide • Aripiprazole • Carpipramine • Clocapramine • Clozapine • Gevotroline • Iloperidone • Melperone • Mosapramine • Olanzapine • Paliperidone • Pimozide • Quetiapine • Risperidone • Sertindole • Ziprasidone • Zotepine; Typical antipsychotics: Loxapine • Pipamperone; Antidepressants: Amitriptyline • Amoxapine • Aptazapine • Etoperidone • Mianserin • Mirtazapine • Nefazodone • Teniloxazine • Trazodone; Others: 5-I-R91150 • AC-90179 • Adatanserin • Altanserin • AMDA • APD-215 • Blonanserin • Cinanserin • CSP-2503 • Cyproheptadine • Deramciclane • Dotarizine • Eplivanserin • Esmirtazapine • Fananserin • Flibanserin • Ketanserin • KML-010 • Lubazodone • Mepiprazole • Metitepine/Methiothepin • Nantenine • Pimavanserin • Pizotifen • Pruvanserin • Rauwolscine • Ritanserin • S-14,671 • Sarpogrelate • Setoperone • Spiperone • Spiramide • SR-46349B • Volinanserin • Xylamidine • YohimbineAgonists: Oxazolines: 4-Methylaminorex • Aminorex; Phenethylamines: Chlorphentermine • Cloforex • DOB • DOC • DOI • DOM • Fenfluramine • MDA • MDMA • Norfenfluramine; Tryptamines: 5-CT • 5-MT • α-Methyl-5-HT; Others: BW-723C86 • Cabergoline • mCPP • Pergolide • PNU-22394 • Ro60-0175
Antagonists: Agomelatine • Asenapine • EGIS-7625 • Ketanserin • Lisuride • LY-272,015 • Metitepine/Methiothepin • PRX-08066 • Rauwolscine • Ritanserin • RS-127,445 • Sarpogrelate • SB-200,646 • SB-204,741 • SB-206,553 • SB-215,505 • SB-221,284 • SB-228,357 • SDZ SER-082 • Tegaserod • YohimbineAgonists: Phenethylamines: 2C-B • 2C-E • 2C-I • 2C-T-2 • 2C-T-7 • 2C-T-21 • DOB • DOC • DOI • DOM • MDA • MDMA • Mescaline; Piperazines: Aripiprazole • mCPP • TFMPP; Tryptamines: 5-CT • 5-MeO-α-ET • 5-MeO-α-MT • 5-MeO-DET • 5-MeO-DiPT • 5-MeO-DMT • 5-MeO-DPT • 5-MT • α-ET • α-Methyl-5-HT • α-MT • Bufotenin • DET • DiPT • DMT • DPT • Psilocin • Psilocybin; Others: A-372,159 • AL-38022A • CP-809,101 • Dimemebfe • Lorcaserin• Medifoxamine • MK-212 • Org 12,962 • ORG-37,684 • Oxaflozane • PNU-22394 • Ro60-0175 • Ro60-0213 • Vabicaserin • WAY-629 • WAY-161,503 • YM-348
Antagonists: Atypical antipsychotics: Clozapine • Iloperidone • Melperone • Olanzapine • Paliperidone • Pimozide • Quetiapine • Risperidone • Sertindole • Ziprasidone • Zotepine; Typical antipsychotics: Chlorpromazine • Loxapine • Pipamperone; Antidepressants: Agomelatine • Amitriptyline • Amoxapine • Aptazapine • Etoperidone • Fluoxetine • Mianserin • Mirtazapine • Nefazodone • Nortriptyline • Tedatioxetine • Trazodone; Others: Adatanserin • Cinanserin • Cyproheptadine • Deramciclane • Dotarizine • Eltoprazine • Esmirtazapine • FR-260,010 • Ketanserin • Ketotifen • Latrepirdine • Metitepine/Methiothepin • Methysergide • Pizotifen • Ritanserin • RS-102,221 • S-14,671 • SB-200,646 • SB-206,553 • SB-221,284 • SB-228,357 • SB-242,084 • SB-243,213 • SDZ SER-082 • Xylamidine5-HT3, 5-HT4, 5-HT5, 5-HT6, 5-HT7 ligands Agonists: Piperazines: BZP • Quipazine; Tryptamines: 2-Methyl-5-HT • 5-CT; Others: Chlorophenylbiguanide • Butanol • Ethanol • Halothane • Isoflurane • RS-56812 • SR-57,227 • SR-57,227-A • Toluene • Trichloroethane • Trichloroethanol • Trichloroethylene • YM-31636
Antagonists: Antiemetics: AS-8112 • Alosetron • Azasetron • Batanopride • Bemesetron • Cilansetron • Dazopride • Dolasetron • Granisetron • Lerisetron • Ondansetron • Palonosetron • Ramosetron • Renzapride • Tropisetron • Zacopride • Zatosetron; Atypical antipsychotics: Clozapine • Olanzapine • Quetiapine; Tetracyclic antidepressants: Amoxapine • Mianserin • Mirtazapine; Others: CSP-2503 • ICS-205,930 • MDL-72,222 • Memantine • Nitrous Oxide • Ricasetron • Sevoflurane • Tedatioxetine • Thujone • Vortioxetine • XenonAgonists: Gastroprokinetic Agents: Cinitapride • Cisapride • Dazopride • Metoclopramide • Mosapride • Prucalopride • Renzapride • Tegaserod • Velusetrag • Zacopride; Others: 5-MT • BIMU8 • CJ-033,466 • PRX-03140 • RS-67333 • RS-67506 • SL65.0155 • Antagonists: GR-113,808 • GR-125,487 • L-Lysine • Piboserod • RS-39604 • RS-67532 • SB-203,186 • SB-204,070Agonists: Lysergamides: Ergotamine • LSD; Tryptamines: 5-CT; Others: Valerenic Acid
Antagonists: Asenapine • Latrepirdine • Metitepine/Methiothepin • Ritanserin • SB-699,551
* Note that the 5-HT5B receptor is not functional in humans.Agonists: Lysergamides: Dihydroergotamine • Ergotamine • Lisuride • LSD • Mesulergine • Metergoline • Methysergide; Tryptamines: 2-Methyl-5-HT • 5-BT • 5-CT • 5-MT • Bufotenin • E-6801 • E-6837 • EMD-386,088 • EMDT • LY-586,713 • Tryptamine; Others: WAY-181,187 • WAY-208,466
Antagonists: Antidepressants: Amitriptyline • Amoxapine • Clomipramine • Doxepin • Mianserin • Nortriptyline; Atypical antipsychotics: Aripiprazole • Asenapine • Clozapine • Fluperlapine • Iloperidone • Olanzapine • Tiospirone; Typical antipsychotics: Chlorpromazine • Loxapine; Others: BGC20-760 • BVT-5182 • BVT-74316 • Cerlapirdine • EGIS-12,233 • GW-742,457 • Ketanserin • Latrepirdine • Lu AE58054 • Metitepine/Methiothepin • MS-245 • PRX-07034 • Ritanserin • Ro04-6790 • Ro 63-0563 • SB-258,585 • SB-271,046 • SB-357,134 • SB-399,885 • SB-742,457Agonists: Lysergamides: LSD; Tryptamines: 5-CT • 5-MT • Bufotenin; Others: 8-OH-DPAT • AS-19 • Bifeprunox • E-55888 • LP-12 • LP-44 • RU-24,969 • Sarizotan
Antagonists: Lysergamides: 2-Bromo-LSD • Bromocriptine • Dihydroergotamine • Ergotamine • Mesulergine • Metergoline • Methysergide; Antidepressants: Amitriptyline • Amoxapine • Clomipramine • Imipramine • Maprotiline • Mianserin; Atypical antipsychotics: Amisulpride • Aripiprazole • Clozapine • Olanzapine • Risperidone • Sertindole • Tiospirone • Ziprasidone • Zotepine; Typical antipsychotics: Chlorpromazine • Loxapine; Others: Butaclamol • EGIS-12,233 • Ketanserin • LY-215,840 • Metitepine/Methiothepin • Pimozide • Ritanserin • SB-258,719 • SB-258,741 • SB-269,970 • SB-656,104 • SB-656,104-A • SB-691,673 • SLV-313 • SLV-314 • Spiperone • SSR-181,507Reuptake inhibitors Selective serotonin reuptake inhibitors (SSRIs): Alaproclate • Citalopram • Dapoxetine • Desmethylcitalopram • Desmethylsertraline • Escitalopram • Femoxetine • Fluoxetine • Fluvoxamine • Indalpine • Ifoxetine • Litoxetine • Lubazodone • Panuramine • Paroxetine • Pirandamine • RTI-353 • Seproxetine • Sertraline • Tedatioxetine • Vilazodone • Vortioxetine • Zimelidine; Serotonin-norepinephrine reuptake inhibitors (SNRIs): Bicifadine • Desvenlafaxine • Duloxetine • Eclanamine • Levomilnacipran • Milnacipran • Sibutramine • Venlafaxine; Serotonin-norepinephrine-dopamine reuptake inhibitors (SNDRIs): Brasofensine • Diclofensine • DOV-102,677 • DOV-21,947 • DOV-216,303 • NS-2359 • SEP-225289 • SEP-227,162 • Tesofensine; Tricyclic antidepressants (TCAs): Amitriptyline • Butriptyline • Cianopramine • Clomipramine • Desipramine • Dosulepin • Doxepin • Imipramine • Lofepramine • Nortriptyline • Pipofezine • Protriptyline • Trimipramine; Tetracyclic antidepressants (TeCAs): Amoxapine; Piperazines: Nefazodone • Trazodone; Antihistamines: Brompheniramine • Chlorphenamine • Diphenhydramine • Mepyramine/Pyrilamine • Pheniramine • Tripelennamine; Opioids: Pethidine • Methadone • Propoxyphene; Others: Cocaine • CP-39,332 • Cyclobenzaprine • Dextromethorphan • Dextrorphan • EXP-561 • Fezolamine • Mesembrine • Nefopam • PIM-35 • Pridefine • Roxindole • SB-649,915 • ZiprasidoneReleasing agents Aminoindanes: 5-IAI • AMMI • ETAI • MDAI • MDMAI • MMAI • TAI; Aminotetralins: 6-CAT • 8-OH-DPAT • MDAT • MDMAT; Oxazolines: 4-Methylaminorex • Aminorex • Clominorex • Fluminorex; Phenethylamines (also Amphetamines, Cathinones, Phentermines, etc): 2-Methyl-MDA • 4-CAB • 4-FA • 4-FMA • 4-HA • 4-MTA • 5-APDB • 5-Methyl-MDA • 6-APDB • 6-Methyl-MDA • AEMMA • Amiflamine • BDB • BOH • Brephedrone • Butylone • Chlorphentermine • Cloforex • Amfepramone • Metamfepramone • DCA • DFMDA • DMA • DMMA • EBDB • EDMA • Ethylone • Etolorex • Fenfluramine (Dexfenfluramine) • Flephedrone • IAP • IMP • Lophophine • MBDB • MDA • MDEA • MDHMA • MDMA • MDMPEA • MDOH • MDPEA • Mephedrone • Methedrone • Methylone • MMA • MMDA • MMDMA • MMMA • NAP • Norfenfluramine • 4-TFMA • pBA • pCA • pIA • PMA • PMEA • PMMA • TAP; Piperazines: 2C-B-BZP • 2-BZP • 3-MeOPP • BZP • DCPP • MBZP • mCPP • MDBZP • MeOPP • Mepiprazole • pCPP • pFPP • pTFMPP • TFMPP; Tryptamines: 4-Methyl-αET • 4-Methyl-αMT • 5-CT • 5-MeO-αET • 5-MeO-αMT • 5-MT • αET • αMT • DMT • Tryptamine (itself); Others: Indeloxazine • Tramadol • ViqualineEnzyme inhibitors AGN-2979 • FenclonineNonselective: Benmoxin • Caroxazone • Echinopsidine • Furazolidone • Hydralazine • Indantadol • Iproclozide • Iproniazid • Isocarboxazid • Isoniazid • Linezolid • Mebanazine • Metfendrazine • Nialamide • Octamoxin • Paraxazone • Phenelzine • Pheniprazine • Phenoxypropazine • Pivalylbenzhydrazine • Procarbazine • Safrazine • Tranylcypromine; MAO-A Selective: Amiflamine • Bazinaprine • Befloxatone • Befol • Brofaromine • Cimoxatone • Clorgiline • Esuprone • Harmala alkaloids (Harmine, Harmaline, Tetrahydroharmine, Harman, Norharman, etc) • Methylene Blue • Metralindole • Minaprine • Moclobemide • Pirlindole • Sercloremine • Tetrindole • Toloxatone • TyrimaOthers Ferrous iron (Fe2+) • Magnesium (Mg2+) • Tetrahydrobiopterin • Vitamin B3 (Niacin, Nicotinamide → NADPH) • Vitamin B6 (Pyridoxine, Pyridoxamine, Pyridoxal → Pyridoxal phosphate) • Vitamin B9 (Folic Acid → Tetrahydrofolic acid) • Vitamin C (Ascorbic acid) • Zinc (Zn2+)OthersTricyclics Classes Acridine • Anthracene • Dibenzazepine • Dibenzocycloheptene • Dibenzodiazepine • Dibenzothiazepine • Dibenzothiepin • Dibenzoxazepine • Dibenzoxepin • Phenothiazine • Pyridazinobenzoxazine • Pyridinobenzodiazepine • ThioxantheneAntidepressants 7-OH-Amoxapine • Amezepine • Amineptine • Amitriptyline • Amitriptylinoxide • Amoxapine • Aptazapine • Azepindole • Azipramine • Butriptyline • Cianopramine • Ciclazindol • Ciclopramine • Clomipramine • Cotriptyline • Cyanodothiepin • Demexiptiline • Depramine/Balipramine • Desipramine • Dibenzepin • Dimetacrine • Dosulepin/Dothiepin • Doxepin • Enprazepine • Esmirtazapine • Fluotracen • Hepzidine • Homopipramol • Imipramine • Imipraminoxide • Intriptyline • Iprindole • Ketipramine • Litracen • Lofepramine • Losindole • Loxapine • Maprotiline • Mariptiline • Mazindol • Melitracen • Metapramine • Mezepine • Mianserin • Mirtazapine • Naranol • Nitroxazepine • Nortriptyline • Noxiptiline • Octriptyline • Opipramol • Oxaprotiline • Pipofezine • Pirandamine • Propizepine • Protriptyline • Quinupramine • Setiptiline/Teciptiline • Tandamine • Tampramine • Tianeptine • Tienopramine • TrimipramineAntihistamines Alimemazine • Azatadine • Clobenzepam • Cyproheptadine • Dacemazine • Deptropine • Desloratadine • Epinastine • Etymemazine • Hydroxyethylpromethazine • Isopromethazine • Isothipendyl • Ketotifen • Latrepirdine • Loratadine • Mebhydrolin • Mequitazine • Methdilazine • Olopatadine • Oxomemazine • Phenindamine • Pimethixene • Promethazine • Propiomazine • Rupatadine • ThiazinamiumAntipsychotics Acetophenazine • Amoxapine • Asenapine • Butaclamol • Butaperazine • Carphenazine • Carpipramine • Chlorpromazine • Chlorprothixene • Ciclindole • Clocapramine • Clomacran • Clotiapine • Clozapine • Flucindole • Fluotracen • Flupentixol • Fluphenazine • Gevotroline • Homopipramol • Levomepromazine/Methotrimeprazine • Loxapine • Maroxepin • Mesoridazine • Metitepine/Methiothepin • Metoxepin • Mosapramine • Naranol • Olanzapine • Perazine • Perphenazine • Periciazine • Piperacetazine • Pipotiazine • Piquindone • Prochlorperazine • Promazine • Prothipendyl • Quetiapine • Sulforidazine • Thiethylperazine • Thiopropazate • Thioridazine • Thiothixene • Trifluoperazine • Triflupromazine • Zotepine • ZuclopenthixolOthers Atiprosin • Carbamazepine • Carvedilol • Cyclobenzaprine • Licarbazepine • Methylene Blue • Monatepil • Oxcarbazepine • Oxitriptyline • Pirenzepine • Pirolate • Pitrazepin • Pizotifen • ProfenamineCategories:- Antiemetics
- Phenothiazines
- Organochlorides
Wikimedia Foundation. 2010.
Look at other dictionaries:
Chlorpromazine — structure de la chlorpromazine Général Nom IUPAC 2 chloro 10 [3( diméthylamino) propyl] … Wikipédia en Français
chlorpromazine — [ klɔrprɔmazin ] n. f. • 1952 aussi chloropromazine; de chlor(o) et prom(eth)azine ♦ Pharm. Médicament de synthèse, tranquillisant qui prévient également les nausées et les vomissements. « Les médicaments qui, telle la chlorpromazine, améliorent… … Encyclopédie Universelle
chlorpromazine — n. a drug derived from phenothiazine and used as a sedative and tranquilizer. [WordNet 1.5] … The Collaborative International Dictionary of English
chlorpromazine — [klôr präm′ə zēn΄] n. [ CHLOR(O) + promazine, C17H20N2S, contr. < promethazine, an antihistaminic drug < PRO(PYL) + (di)meth(ylamine), a gaseous compound, (CH3)2NH + (phenothi)azine, C12H9NS < PHENO + THIAZINE] a synthetic drug,… … English World dictionary
chlorpromazine — /klawr prom euh zeen , klohr /, n. a grayish white, crystalline powder, C17H19ClN2S, derived from phenothiazine, used chiefly to inhibit nausea and vomiting and as a major tranquilizer in the management of schizophrenia and related psychoses.… … Universalium
chlorpromazine — A phenothiazine antipsychotic agent with antiemetic, antiadrenergic, and anticholinergic actions. c. hydrochloride c. suitable for oral, intramuscular, and intravenous administration. * * * chlor·prom·a·zine … Medical dictionary
chlorpromazine — n. a phenothiazine antipsychotic drug used in the treatment of schizophrenia and mania; it is also used to control nausea and vomiting in terminal illness. Chlorpromazine is administered by mouth or injection or as a rectal suppository; common… … The new mediacal dictionary
Chlorpromazine (Maladie Professionnelle) — Cet article décrit les critères administratifs pour qu une allergie à la chlorpromazine soit reconnue comme maladie professionnelle. Cet article relève du domaine de la législation sur la protection sociale et a un caractère davantage juridique… … Wikipédia en Français
Chlorpromazine (maladie professionnelle) — Cet article décrit les critères administratifs pour qu une allergie à la chlorpromazine soit reconnue comme maladie professionnelle. Cet article relève du domaine de la législation sur la protection sociale et a un caractère davantage juridique… … Wikipédia en Français
Chlorpromazine and the Phenothiazine Antipsychotics — (from 1952). Prehistory: In 1883, August Bernthsen (1855–1931), a postdoctoral student in chemistry at Heidelberg, synthesized a hydrocarbon molecule with two benzol rings connected to each other by a sulfur and a nitrogen atom. Writing in… … Historical dictionary of Psychiatry