- Pralidoxime
-
Pralidoxime 
Systematic (IUPAC) name 2-[(hydroxyimino)methyl]-1-methylpyridin-1-ium Clinical data AHFS/Drugs.com Micromedex Detailed Consumer Information Pregnancy cat. C Legal status RX-only Identifiers CAS number 6735-59-7 
ATC code V03AB04 PubChem CID 6789253 DrugBank APRD01193 ChemSpider 5193737 
UNII P7MU9UTP52 
KEGG C07400 
ChEBI CHEBI:8354 
ChEMBL CHEMBL1420 
Synonyms 1-methylpyridine-6-carbaldehyde oxime Chemical data Formula C7H9N2O+ Mol. mass 137.159 g/mol SMILES eMolecules & PubChem  (what is this?) (verify)
(what is this?) (verify)Pralidoxime (2-pyridine aldoxime methyl chloride,) or 2-PAM, usually as the chloride or methiodide salts, belongs to a family of compounds called oximes that bind to organophosphate-inactivated acetylcholinesterase. It is used to combat poisoning by organophosphates[1] or acetylcholinesterase inhibitors (nerve agents), in conjunction with atropine and diazepam. In India, it is marketed by Nucleus Inc. with the brand names LyoPAM and PurePAM.
Contents
Mechanism of action
Pralidoxime, typically used in cases of organophosphate poisoning (causes ACHase inhibition), attaches to the site where a cholinesterase inhibitor has attached, then attaches to the inhibitor, removing the organophosphate from cholinesterase, allowing it to work normally again. This is known as "regenerating" or "reactivating" acetylcholinesterase allowing the breakdown of Ach at the synapse. After some time, though, some inhibitors can develop a permanent bond with cholinesterase, known as aging, where oximes such as pralidoxime can not reverse the bond.[2] Pralidoxime is often used with atropine (a muscarinic antagonist) to help reduce the parasympathetic effects of organophosphate poisoning. Pralidoxime can also be used to treat neostigmine or pyridostigmine (both ACHase inhibitors) overdoses due to it's ACHase inhibitor regenerating capacities.
Pralidoxime has an important role in reversing paralysis of the respiratory muscles but due to its poor blood-brain barrier penetration, it has little effect on centrally-mediated respiratory depression. This is why atropine which has excellent blood-brain barrier penetration, is concomitantly administered with pralidoxime during the treatment of organophosphate poisoning.
Dosage
- Adults: 30 mg/kg (typically 1-2 g), administered by intravenous therapy over 15–30 minutes or intramuscular injection or subcutaneous injection, repeated 60 minutes later. It can also be given as a 500 mg/hr continuous IV infusion.
- Children: 20–50 mg/kg followed by a maintenance infusion at 5–10 mg/kg/hr.
Intravenous infusions can lead to respiratory or cardiac arrest if given too quickly.[3]
Interactions
When atropine and pralidoxime are used together, the signs of atropinization (flushing, mydriasis, tachycardia, dryness of the mouth and nose) may occur earlier than might be expected when atropine is used alone. This is especially true if the total dose of atropine has been large and the administration of pralidoxime has been delayed.
The following precautions should be kept in mind in the treatment of anticholinesterase poisoning, although they do not bear directly on the use of pralidoxime: since barbiturates are potentiated by the anticholinesterases, they should be used cautiously in the treatment of convulsions; morphine, theophylline, aminophylline, succinylcholine, reserpine, and phenothiazine-type tranquilizers should be avoided in patients with organophosphate poisoning.
Contraindications
There are no known absolute contraindications for the use of pralidoxime. Relative contraindications include known hypersensitivity to the drug and other situations in which the risk of its use clearly outweighs possible benefit.
Chemistry
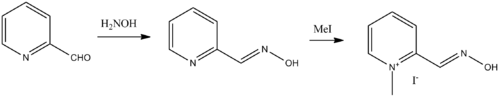
Pralidoxime, 2-pyridinaldoxime methylchloride, is synthesized by reacting picolinaldehyde (2-formyl pyridine) with hydroxylamine, giving pyridine-2-aldoxime, which is further reacted with methyl iodide, giving the desired pralidoxime.
- D. Nachmansonn, S. Ginsburg, U.S. Patent 2,816,113 (1957).
- L.P. Black, U.S. Patent 3,123,613 (1964).
- D.E. Easterday, A.A. Kondritzer, U.S. Patent 3,140,289 (1964).
- W.B. McDowell, U.S. Patent 3,155,674 (1964).
See also
References
- ^ Jokanović M, Prostran M (2009). "Pyridinium oximes as cholinesterase reactivators. Structure-activity relationship and efficacy in the treatment of poisoning with organophosphorus compounds". Curr. Med. Chem. 16 (17): 2177–88. doi:10.2174/092986709788612729. PMID 19519385. http://www.bentham-direct.org/pages/content.php?CMC/2009/00000016/00000017/0004C.SGM.
- ^ http://www.atsdr.cdc.gov/csem/cholinesterase/pam_medications.html
- ^ Baxter Healthcare Corporation 2006, Protopam Prescribing Information
External links
Antidotes (V03AB) Nervous system Barbiturate overdoseBemegride • EthamivanBenzodiazepine overdoseGHB overdoseReversal of neuromuscular blockadeCardiovascular Other Paracetamol toxicity (Acetaminophen)OtherPrednisolone/promethazine • oxidizing agent (potassium permanganate) • iodine-131 (Potassium iodide) • Methylthioninium chloride#Emetic Ipecacuanha (Syrup of ipecac) • Copper sulfateCholinergics Receptor ligands Agonists: 77-LH-28-1 • AC-42 • AC-260,584 • Aceclidine • Acetylcholine • AF30 • AF150(S) • AF267B • AFDX-384 • Alvameline • AQRA-741 • Arecoline • Bethanechol • Butyrylcholine • Carbachol • CDD-0034 • CDD-0078 • CDD-0097 • CDD-0098 • CDD-0102 • Cevimeline • cis-Dioxolane • Ethoxysebacylcholine • LY-593,039 • L-689,660 • LY-2,033,298 • McNA343 • Methacholine • Milameline • Muscarine • NGX-267 • Ocvimeline • Oxotremorine • PD-151,832 • Pilocarpine • RS86 • Sabcomeline • SDZ 210-086 • Sebacylcholine • Suberylcholine • Talsaclidine • Tazomeline • Thiopilocarpine • Vedaclidine • VU-0029767 • VU-0090157 • VU-0152099 • VU-0152100 • VU-0238429 • WAY-132,983 • Xanomeline • YM-796
Antagonists: 3-Quinuclidinyl Benzilate • 4-DAMP • Aclidinium Bromide • Anisodamine • Anisodine • Atropine • Atropine Methonitrate • Benactyzine • Benzatropine (Benztropine) • Benzydamine • BIBN 99 • Biperiden • Bornaprine • CAR-226,086 • CAR-301,060 • CAR-302,196 • CAR-302,282 • CAR-302,368 • CAR-302,537 • CAR-302,668 • CS-27349 • Cyclobenzaprine • Cyclopentolate • Darifenacin • DAU-5884 • Dimethindene • Dexetimide • DIBD • Dicyclomine (Dicycloverine) • Ditran • EA-3167 • EA-3443 • EA-3580 • EA-3834 • Elemicin • Etanautine • Etybenzatropine (Ethylbenztropine) • Flavoxate • Himbacine • HL-031,120 • Ipratropium bromide • J-104,129 • Hyoscyamine • Mamba Toxin 3 • Mamba Toxin 7 • Mazaticol • Mebeverine • Methoctramine • Metixene • Myristicin • N-Ethyl-3-Piperidyl Benzilate • N-Methyl-3-Piperidyl Benzilate • Orphenadrine • Otenzepad • Oxybutynin • PBID • PD-102,807 • PD-0298029 • Phenglutarimide • Phenyltoloxamine • Pirenzepine • Piroheptine • Procyclidine • Profenamine • RU-47,213 • SCH-57,790 • SCH-72,788 • SCH-217,443 • Scopolamine (Hyoscine) • Solifenacin • Telenzepine • Tiotropium bromide • Tolterodine • Trihexyphenidyl • Tripitamine • Tropatepine • Tropicamide • WIN-2299 • Xanomeline • Zamifenacin; Others: 1st Generation Antihistamines (Brompheniramine, chlorphenamine, cyproheptadine, dimenhydrinate, diphenhydramine, doxylamine, mepyramine/pyrilamine, phenindamine, pheniramine, tripelennamine, triprolidine, etc) • Tricyclic Antidepressants (Amitriptyline, doxepin, trimipramine, etc) • Tetracyclic Antidepressants (Amoxapine, maprotiline, etc) • Typical Antipsychotics (Chlorpromazine, thioridazine, etc) • Atypical Antipsychotics (Clozapine, olanzapine, quetiapine, etc)Agonists: 5-HIAA • A-84,543 • A-366,833 • A-582,941 • A-867,744 • ABT-202 • ABT-418 • ABT-560 • ABT-894 • Acetylcholine • Altinicline • Anabasine • Anatoxin-a • AR-R17779 • Butyrylcholine • Carbachol • Cotinine • Cytisine • Decamethonium • Desformylflustrabromine • Dianicline • Dimethylphenylpiperazinium • Epibatidine • Epiboxidine • Ethanol • Ethoxysebacylcholine • EVP-4473 • EVP-6124 • Galantamine • GTS-21 • Ispronicline • Lobeline • MEM-63,908 (RG-3487) • Nicotine • NS-1738 • PHA-543,613 • PHA-709,829 • PNU-120,596 • PNU-282,987 • Pozanicline • Rivanicline • Sazetidine A • Sebacylcholine • SIB-1508Y • SIB-1553A • SSR-180,711 • Suberylcholine • TC-1698 • TC-1734 • TC-1827 • TC-2216 • TC-5214 • TC-5619 • TC-6683 • Tebanicline • Tropisetron • UB-165 • Varenicline • WAY-317,538 • XY-4083
Antagonists: 18-Methoxycoronaridine • α-Bungarotoxin • α-Conotoxin • Alcuronium • Amantadine • Anatruxonium • Atracurium • Bupropion (Amfebutamone) • Chandonium • Chlorisondamine • Cisatracurium • Coclaurine • Coronaridine • Dacuronium • Decamethonium • Dextromethorphan • Dextropropoxyphene • Dextrorphan • Diadonium • DHβE • Dimethyltubocurarine (Metocurine) • Dipyrandium • Dizocilpine (MK-801) • Doxacurium • Duador • Esketamine • Fazadinium • Gallamine • Hexafluronium • Hexamethonium (Benzohexonium) • Ibogaine • Isoflurane • Ketamine • Kynurenic acid • Laudexium (Laudolissin) • Levacetylmethadol • Malouetine • Mecamylamine • Memantine • Methadone • Methorphan (Racemethorphan) • Methyllycaconitine • Metocurine • Mivacurium • Morphanol (Racemorphanol) • Neramexane • Nitrous Oxide • Pancuronium • Pempidine • Pentamine • Pentolinium • Phencyclidine • Pipecuronium • Radafaxine • Rapacuronium • Rocuronium • Surugatoxin • Suxamethonium (Succinylcholine) • Thiocolchicoside • Toxiferine • Trimethaphan • Tropeinium • Tubocurarine • Vecuronium • XenonReuptake inhibitors PlasmalemmalCHT InhibitorsHemicholinium-3 (Hemicholine; HC3) • TriethylcholineVAChT InhibitorsEnzyme inhibitors ChAT inhibitors1-(-Benzoylethyl)pyridinium • 2-(α-Naphthoyl)ethyltrimethylammonium • 3-Chloro-4-stillbazole • 4-(1-Naphthylvinyl)pyridine • Acetylseco hemicholinium-3 • Acryloylcholine • AF64A • B115 • BETA • CM-54,903 • CatabolismAChE inhibitorsReversible: Carbamates: Aldicarb • Bendiocarb • Bufencarb • Carbaryl • Carbendazim • Carbetamide • Carbofuran • Chlorbufam • Chloropropham • Ethienocarb • Ethiofencarb • Fenobucarb • Fenoxycarb • Formetanate • Furadan • Ladostigil • Methiocarb • Methomyl • Miotine • Oxamyl • Phenmedipham • Pinmicarb • Pirimicarb • Propamocarb • Propham • Propoxur; Stigmines: Ganstigmine • Neostigmine • Phenserine • Physostigmine • Pyridostigmine • Rivastigmine; Others: Acotiamide • Ambenonium • Donepezil • Edrophonium • Galantamine • Huperzine A • Minaprine • Tacrine • Zanapezil
Irreversible: Organophosphates: Acephate • Azinphos-methyl • Bensulide • Cadusafos • Chlorethoxyfos • Chlorfenvinphos • Chlorpyrifos • Chlorpyrifos-Methyl • Coumaphos • Cyclosarin (GF) • Demeton • Demeton-S-Methyl • Diazinon • Dichlorvos • Dicrotophos • Diisopropyl fluorophosphate (Guthion) • Diisopropylphosphate • Dimethoate • Dioxathion • Disulfoton • EA-3148 • Echothiophate • Ethion • Ethoprop • Fenamiphos • Fenitrothion • Fenthion • Fosthiazate • GV • Isofluorophate • Isoxathion • Malaoxon • Malathion • Methamidophos • Methidathion • Metrifonate • Mevinphos • Monocrotophos • Naled • Novichok agent • Omethoate • Oxydemeton-Methyl • Paraoxon • Parathion • Parathion-Methyl • Phorate • Phosalone • Phosmet • Phostebupirim • Phoxim • Pirimiphos-Methyl • Sarin (GB) • Soman (GD) • Tabun (GA) • Temefos • Terbufos • Tetrachlorvinphos • Tribufos • Trichlorfon • VE • VG • VM • VR • VX; Others: Demecarium • Onchidal (Onchidella binneyi)BChE inhibitorsCymserine * Many of the acetylcholinesterase inhibitors listed above act as butyrylcholinesterase inhibitors.Others Choline (Lecithin) • Citicoline • Cyprodenate • Dimethylethanolamine (DMAE, deanol) • Glycerophosphocholine • Meclofenoxate (Centrophenoxine) • Phosphatidylcholine • Phosphatidylethanolamine • Phosphorylcholine • PirisudanolOthersAcetylcholine releasing agents: α-Latrotoxin • β-Bungarotoxin; Acetylcholine release inhibitors: Botulinum toxin (Botox); Acetylcholinesterase reactivators: Asoxime • Obidoxime • PralidoximeCategories:- Antidotes
- Oximes
- Quaternary ammonium compounds
Wikimedia Foundation. 2010.
Look at other dictionaries:
pralidoxime chloride — Used to restore the inactivated cholinesterase activity resulting from organophosphate poisoning; has some limited value as an antagonist of the carbamate type of cholinesterase inhibitors that are used in the treatment of myasthenia gravis.… … Medical dictionary
pralidoxime — noun An oxime used in conjunction with atropine to combat poisoning by organophosphates or acetylcholinesterase inhibitors (nerve agents) … Wiktionary
pralidoxime — pral·i·dox·ime .pral i däk .sēm n a substance C7H9ClN2O that restores the reactivity of cholinesterase and is used to counteract phosphorylation (as by an organophosphate pesticide) called also 2 PAM see PROTOPAM * * * pral·i·dox·ime… … Medical dictionary
pralidoxime — … Useful english dictionary
2-pralidoxime — One of several oximes that are effective in reversing cholinesterase inhibition by organophosphates. The 2 PAM facilitates the hydrolysis of the phosphorylated enzyme so as to regenerate active cholinesterase … Medical dictionary
Nerve agent — This article is about the chemical. For the band, see The Nerve Agents. This article forms part of the series Chemical agents Lethal agents Blood agents Cyanogen chloride (CK) … Wikipedia
Sarin — For other uses, see Sarin (disambiguation). Not to be confused with Serine. Sarin[1] … Wikipedia
Atropine — Systematic (IUPAC) name … Wikipedia
VX (nerve agent) — Chembox new ImageFile = VX S enantiomer 2D skeletal.png ImageName = Skeletal formula of the S enantiomer of VX ImageFile1 = VX S enantiomer 3D balls.png ImageName1 = Ball and stick model of the S enantiomer of VX IUPACName = Ethyl { [2 [di(propan … Wikipedia
2-PAM — Abbreviation for 2 pralidoxime. * * * 2 PAM .tü .pē .ā em n PRALIDOXIME * * * pralidoxime … Medical dictionary