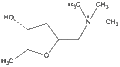- Muscarine
-
Muscarine 
 2,5-anhydro-1,4,6-trideoxy-6-(trimethylammonio)-D-ribo-hexitolOther namesL-(+)-muscarine, muscarin, (2S,4R,5S)-(4-hydroxy-5-methyl-tetrahydrofuran-2-ylmethyl)-trimethyl-ammonium
2,5-anhydro-1,4,6-trideoxy-6-(trimethylammonio)-D-ribo-hexitolOther namesL-(+)-muscarine, muscarin, (2S,4R,5S)-(4-hydroxy-5-methyl-tetrahydrofuran-2-ylmethyl)-trimethyl-ammoniumIdentifiers CAS number 300-54-9 PubChem 9308 ChemSpider 8949 
ChEMBL CHEMBL12587 
Jmol-3D images Image 1 - O[C@@H]1C[C@H](O[C@H]1C)C[N+](C)(C)C
Properties Molecular formula C9H20NO2+ Molar mass 174.26 g/mol  (verify) (what is:
(verify) (what is:  /
/ ?)
?)
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references Muscarine, L-(+)-muscarine, or muscarin is a natural product found in certain mushrooms, particularly in Inocybe and Clitocybe species, such as the deadly C. dealbata. Mushrooms in the genera Entoloma and Mycena have also been found to contain levels of muscarine which can be dangerous if ingested. Muscarine has been found in harmless trace amounts in Boletus, Hygrocybe, Lactarius and Russula. Muscarine is only a trace compound in the fly agaric Amanita muscaria; the pharmacologically more relevant compound from this mushroom is muscimol. The A. muscaria contains a variable dose of muscarine, usually around 0.0003% fresh weight. This is very low and toxicity symptoms occur very rarely. Inocybe and Clitocybe contain muscarine concentrations up to 1.6%.[1]
Contents
History
Muscarine was first isolated from Amanita muscaria in 1869. It was the first parasympathomimetic substance ever studied and causes profound activation of the peripheral parasympathetic nervous system that may end in convulsions and death. Being a quaternary amine, muscarine is less completely absorbed from the gastrointestinal tract than tertiary amines, but it does cross the blood brain barrier.[2] Muscarinic agonists activate muscarinic receptors while nicotinic agonists activate nicotin receptors. Both are direct-acting cholinomimetics; they produce their effects by binding to and activating cholinergic receptors. Final proof of the structure was given by Jellinek (61) in 1957 with the help of X-ray diffraction analysis. These new findings set into motion research not only on the pharmacology of muscarine, but also on that of muscarine-like substances that are structurally related to acetylcholine.
Structure and reactivity
‘Muscarine mimics the function of the natural neurotransmitter acetylcholine in the muscarinic part of the cholinergic nervous system.’ Despite of the less flexible structure due to the five-membered ring in the molecular skeleton.[3]
There are two mirror forms of muscarin, named: 2S-muscarine and 2R-muscarine.
Efficient synthesis of (+)-muscarine
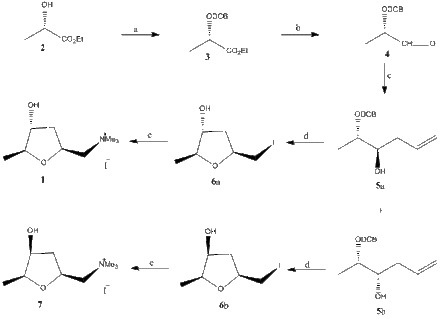
The next is a very efficient way of synthesis of (+)-muscarine according to the scientists Chan and Li in the Canadian journal of Chemistry in 1992.[4] S-(-)-Ethyl lactate (2)(figure 3) is converted into the 2,6-dichlorobenzyl ether (3). Diisobutylaluminium hydride (DIBAL) reduction of the 2,6-dichlorobenzyl ether gives the aldehyde (4). Treatment of the crude aldehyde with allyl bromide and zinc powder in water with NH4Cl as catalyst resulted in an anti:syn mixture of 5a and 5b. Treatment of 5a with iodine in CH3CN at 0°C gives the cyclized product 6a. Finally treatment of 6a with excess trimethylamine in ethanol gave (+)-muscarine (2S,4R,5S). A similar reaction sequence with 5b gave (+)-epimuscarine (7). [5]
Mechanism of action
Muscarine mimics the action of the neurotransmitter acetylcholine by binding muscarinic acetylcholine receptors. These receptors were named after muscarine. There are 5 different types of muscarinic receptors; M1 - M5, and most tissues express a mixture of subtypes. The M2 and M3 subtypes mediate muscarinic responses at peripheral autonomic tissues. M1 and M4 subtypes are more abundant in brain and autonomic ganglia. M1, M3 and M5 interact with Gq proteins to stimulate phosphoinositide hydrolysis and the release of intracellular calcium. M2 and M4 receptors interact with Gi proteins to inhibit adenylyl cyclase, which results in a decrease of intracellular concentration of cyclic adenosine monophosphate (cAMP). Most agonists for muscarine receptors are not selective for subtypes.[6]
Metabolism
A paucity of research exists on the metabolism of muscarine in the human body, suggesting this compound is not metabolized. Though there has been extensive research in the field of acetylcholine metabolism by acetylcholinesterase, muscarine is not metabolized by this enzyme, partly explaining the compound's toxicity. Muscarine is readily soluble in water. The most likely way to leave the blood is via renal clearance and it will eventually leave the body in urine.[7]
Drug
Muscarinic agonists are used as drugs in treating glaucoma, postoperative ileus, congenital megacolon, urinary retention and Xerostomia. Muscarine is contraindicated in patients with diseases that make them susceptible to parasympathetic stimulation, patients who have asthma or COPD or patients who have peptic ulcer disease. Also patients with an obstruction in the gastrointestinal or urinary tract are not prescribed muscarine because it will aggravate the obstruction, causing pressure to build up that may lead to perforation. The applications of muscarine to the treatment of disease are yet in their infancy. The physiological effects indicate the direction of the remedial applications. As muscarine stimulates so powerfully the muscular fiber of the intestine, and the secretions of the pancreas, liver, and intestinal mucous membrane, it ought to be very serviceable in cases of constipation with torpor of the organs concerned in digestion. When constipation is due to paresis of the muscular layer of the bowel and to deficient secretion, this remedy will probably relieve it. Muscarine can also be used in combination with other remedies for the treatment of intestinal torpor and deficient secretion. Muscarine is of doubtful propriety, if not positively contra-indicated, in renal affections characterized by deficiency in the excretion. On the other hand, it ought to be of signal service in diabetes insipidus and in saccharine diabetes. It has been used successfully to arrest the secretion of milk.[8]
Efficacy
As muscarine works on the muscarinic acetylcholine receptor the best comparison can be made with acetylcholine, which normally works on this receptor. Pure muscarine compared to pure acetylcholine is stated in most cases to be more potent, its actions always slower but longer lasting then acetylcholine. A possible explanation for this long lasting behavior might be that muscarine doesn’t get hydrolyzed by acetylcholinesterase in the synaptic cleft.[9]
Toxicology
Muscarine poisoning is characterized by miosis, blurred vision, increased salivation, excessive sweating, lacrimation, bronchial secretions, bronchoconstriction, bradycardia, abdominal cramping, increased gastic acid secretion, diarrhea and polyuria. If muscarine reaches the brain it can cause tremor, convulsions and hypothermia. Cardiac ventricles contain muscarinic receptors that mediate a decrease in the force of contractions leading to a lower blood pressure. If muscarine is administered intravenously, muscarine can trigger acute circulatory failure with cardiac arrest.[10] The symptoms of intoxication with mushrooms rich in muscarine, especially Inocybe, are very typical: The symptoms start early, after one-quarter to two hours, with headache, nausea, vomiting, and constriction of the pharynx. Then salivation, lacrimation, and diffuse perspiration set in, combined with miosis, disturbed accommodation, and reduced vision. Gastric and small bowel colic leads to diarrhea, and there is a painful urge for urination. Bronchoconstriction leads to asthmatic attacks and severe dyspnea, and bradycardia combined with marked hypotension and vasodilation results in circulatory shock. Death after 8 to 9 hours has been reported in about 5 % of the cases, but can be avoided completely by prompt diagnosis and treatment with atropine.[11]
Antidote
The specific antidote is atropine. Atropine is also an alkaloid and inhibits acetylcholine and thus muscarine by binding to muscarinic receptors. Other muscarinic antagonists are scopolamine and pirenzepine. Muscarinic antagonists dilate the pupil and relax the ciliary muscle, are used in treatment of inflammatory uveitis and it's associated with glaucoma. They are also used to treat urinary incontinence and diseases characterized by bowel hypermotility such as irritable bowel syndrome. Muscarinic antagonists are often called parasympatholytics because they have the same effect as agents that block postganglionic parasympatic nerves.
See also
References
- ^ Lurie, Y., Wasser, S.P., Taha, M., Shehade, H., Nijim, J., et al (2009). "Mushroom poisoning from species of genus Inocybe (fiber head mushroom): a case series with exact species identification " Clinical toxicology 47(6): 562-565.
- ^ Pappano Achilles J, "Chapter 7. Cholinoceptor-Activating & Cholinesterase-Inhibiting Drugs" (Chapter). Katzung BG: Basic & Clinical Pharmacology, 11e
- ^ Frydenvang, K., Jensen, B. (1993) Acta Crystallographica Section C-Crystal Structure Communications Structures of muscarine picrate and muscarine tetraphenylborate 49, 985-990
- ^ Chan, T. H., Li, C.J. (1992). "A Concise synthesis of (+)-muscarine." Canadian Journal of Chemistry-revue 70(11): 2726-2729
- ^ Chan, T. H., Li, C.J. (1992). "A Concise synthesis of (+)-muscarine." Canadian Journal of Chemistry-revue 70(11): 2726-2729
- ^ Human Pharmacology, Molecular to Clinical, Chapter 9, Third Edition, Brody, Larner, Minneman
- ^ Roberts Bartholow, “A practical treatise on materia medica and therapeutics”, 1908, isbn: 9781143467677,
- ^ Roberts Bartholow; "A Practical Treatise On Materia Medica And Therapeutics"; 1908, Appleton And Company
- ^ Fraser, P.J.,"Pharmacological actions of pure muscarine chloride", Brit. J. Pharmacol. (1957), 12, 47.:page 51
- ^ Lurie, Y., Wasser, S., Taha, M., Shehade, H., Nijim, J., Hoffmann, Y, Basis, F, Vardi, M., Lavon, O., Suaed, S., Bisharat, B, and Bentur, Y. (2009) Mushroom poisoning from species of genus Inocybe (fiber head mushroom): a case series with exact species identification, Clinical Toxicology vol. 47 no. 6
- ^ Peter G. Waser; Chemistry and pharmacology of muscarine, muscarone and some related compounds; Pharmacology Department, University of Zurich, Switzerland 1961
External links
A. albocreata • A. crenulata • A. farinosa • A. frostiana • A. gemmata • A. multisquamosa • A. muscaria • A. pantherina • A. porphyria • A. regalis • A. strobiliformis • A. xanthocephala Compounds
Destroying angels A. bisporigera • A. exitialis • A. magnivelaris • A. ocreata • A. verna • A. virosa • A. virosiformisOther membersA. arocheae • A. phalloides • A. subjunquileaCompoundsPhallacidin • Phallacin • Phallisacin • Phallisin • Phalloidin • Phalloin • ProphalloinOther compoundsAntamanide • Phallolysin • ToxophallinA. abrupta • A. nauseosa • A. proxima • A. smithiana • A. sphaerobulbosa • A. thiersii CompoundsAllenic norleucine (2-amino-4,5-hexadienoic acid) • PropargylglycineA. rubescens (A. amerirubescens nom. prov.) CompoundsRubescenslysinCholinergics Receptor ligands Agonists: 77-LH-28-1 • AC-42 • AC-260,584 • Aceclidine • Acetylcholine • AF30 • AF150(S) • AF267B • AFDX-384 • Alvameline • AQRA-741 • Arecoline • Bethanechol • Butyrylcholine • Carbachol • CDD-0034 • CDD-0078 • CDD-0097 • CDD-0098 • CDD-0102 • Cevimeline • cis-Dioxolane • Ethoxysebacylcholine • LY-593,039 • L-689,660 • LY-2,033,298 • McNA343 • Methacholine • Milameline • Muscarine • NGX-267 • Ocvimeline • Oxotremorine • PD-151,832 • Pilocarpine • RS86 • Sabcomeline • SDZ 210-086 • Sebacylcholine • Suberylcholine • Talsaclidine • Tazomeline • Thiopilocarpine • Vedaclidine • VU-0029767 • VU-0090157 • VU-0152099 • VU-0152100 • VU-0238429 • WAY-132,983 • Xanomeline • YM-796
Antagonists: 3-Quinuclidinyl Benzilate • 4-DAMP • Aclidinium Bromide • Anisodamine • Anisodine • Atropine • Atropine Methonitrate • Benactyzine • Benzatropine (Benztropine) • Benzydamine • BIBN 99 • Biperiden • Bornaprine • CAR-226,086 • CAR-301,060 • CAR-302,196 • CAR-302,282 • CAR-302,368 • CAR-302,537 • CAR-302,668 • CS-27349 • Cyclobenzaprine • Cyclopentolate • Darifenacin • DAU-5884 • Dimethindene • Dexetimide • DIBD • Dicyclomine (Dicycloverine) • Ditran • EA-3167 • EA-3443 • EA-3580 • EA-3834 • Elemicin • Etanautine • Etybenzatropine (Ethylbenztropine) • Flavoxate • Himbacine • HL-031,120 • Ipratropium bromide • J-104,129 • Hyoscyamine • Mamba Toxin 3 • Mamba Toxin 7 • Mazaticol • Mebeverine • Methoctramine • Metixene • Myristicin • N-Ethyl-3-Piperidyl Benzilate • N-Methyl-3-Piperidyl Benzilate • Orphenadrine • Otenzepad • Oxybutynin • PBID • PD-102,807 • PD-0298029 • Phenglutarimide • Phenyltoloxamine • Pirenzepine • Piroheptine • Procyclidine • Profenamine • RU-47,213 • SCH-57,790 • SCH-72,788 • SCH-217,443 • Scopolamine (Hyoscine) • Solifenacin • Telenzepine • Tiotropium bromide • Tolterodine • Trihexyphenidyl • Tripitamine • Tropatepine • Tropicamide • WIN-2299 • Xanomeline • Zamifenacin; Others: 1st Generation Antihistamines (Brompheniramine, chlorphenamine, cyproheptadine, dimenhydrinate, diphenhydramine, doxylamine, mepyramine/pyrilamine, phenindamine, pheniramine, tripelennamine, triprolidine, etc) • Tricyclic Antidepressants (Amitriptyline, doxepin, trimipramine, etc) • Tetracyclic Antidepressants (Amoxapine, maprotiline, etc) • Typical Antipsychotics (Chlorpromazine, thioridazine, etc) • Atypical Antipsychotics (Clozapine, olanzapine, quetiapine, etc)Agonists: 5-HIAA • A-84,543 • A-366,833 • A-582,941 • A-867,744 • ABT-202 • ABT-418 • ABT-560 • ABT-894 • Acetylcholine • Altinicline • Anabasine • Anatoxin-a • AR-R17779 • Butyrylcholine • Carbachol • Cotinine • Cytisine • Decamethonium • Desformylflustrabromine • Dianicline • Dimethylphenylpiperazinium • Epibatidine • Epiboxidine • Ethanol • Ethoxysebacylcholine • EVP-4473 • EVP-6124 • Galantamine • GTS-21 • Ispronicline • Lobeline • MEM-63,908 (RG-3487) • Nicotine • NS-1738 • PHA-543,613 • PHA-709,829 • PNU-120,596 • PNU-282,987 • Pozanicline • Rivanicline • Sazetidine A • Sebacylcholine • SIB-1508Y • SIB-1553A • SSR-180,711 • Suberylcholine • TC-1698 • TC-1734 • TC-1827 • TC-2216 • TC-5214 • TC-5619 • TC-6683 • Tebanicline • Tropisetron • UB-165 • Varenicline • WAY-317,538 • XY-4083
Antagonists: 18-Methoxycoronaridine • α-Bungarotoxin • α-Conotoxin • Alcuronium • Amantadine • Anatruxonium • Atracurium • Bupropion (Amfebutamone) • Chandonium • Chlorisondamine • Cisatracurium • Coclaurine • Coronaridine • Dacuronium • Decamethonium • Dextromethorphan • Dextropropoxyphene • Dextrorphan • Diadonium • DHβE • Dimethyltubocurarine (Metocurine) • Dipyrandium • Dizocilpine (MK-801) • Doxacurium • Duador • Esketamine • Fazadinium • Gallamine • Hexafluronium • Hexamethonium (Benzohexonium) • Ibogaine • Isoflurane • Ketamine • Kynurenic acid • Laudexium (Laudolissin) • Levacetylmethadol • Malouetine • Mecamylamine • Memantine • Methadone • Methorphan (Racemethorphan) • Methyllycaconitine • Metocurine • Mivacurium • Morphanol (Racemorphanol) • Neramexane • Nitrous Oxide • Pancuronium • Pempidine • Pentamine • Pentolinium • Phencyclidine • Pipecuronium • Radafaxine • Rapacuronium • Rocuronium • Surugatoxin • Suxamethonium (Succinylcholine) • Thiocolchicoside • Toxiferine • Trimethaphan • Tropeinium • Tubocurarine • Vecuronium • XenonReuptake inhibitors PlasmalemmalCHT InhibitorsHemicholinium-3 (Hemicholine; HC3) • TriethylcholineVAChT InhibitorsEnzyme inhibitors ChAT inhibitors1-(-Benzoylethyl)pyridinium • 2-(α-Naphthoyl)ethyltrimethylammonium • 3-Chloro-4-stillbazole • 4-(1-Naphthylvinyl)pyridine • Acetylseco hemicholinium-3 • Acryloylcholine • AF64A • B115 • BETA • CM-54,903 • CatabolismAChE inhibitorsReversible: Carbamates: Aldicarb • Bendiocarb • Bufencarb • Carbaryl • Carbendazim • Carbetamide • Carbofuran • Chlorbufam • Chloropropham • Ethienocarb • Ethiofencarb • Fenobucarb • Fenoxycarb • Formetanate • Furadan • Ladostigil • Methiocarb • Methomyl • Miotine • Oxamyl • Phenmedipham • Pinmicarb • Pirimicarb • Propamocarb • Propham • Propoxur; Stigmines: Ganstigmine • Neostigmine • Phenserine • Physostigmine • Pyridostigmine • Rivastigmine; Others: Acotiamide • Ambenonium • Donepezil • Edrophonium • Galantamine • Huperzine A • Minaprine • Tacrine • Zanapezil
Irreversible: Organophosphates: Acephate • Azinphos-methyl • Bensulide • Cadusafos • Chlorethoxyfos • Chlorfenvinphos • Chlorpyrifos • Chlorpyrifos-Methyl • Coumaphos • Cyclosarin (GF) • Demeton • Demeton-S-Methyl • Diazinon • Dichlorvos • Dicrotophos • Diisopropyl fluorophosphate (Guthion) • Diisopropylphosphate • Dimethoate • Dioxathion • Disulfoton • EA-3148 • Echothiophate • Ethion • Ethoprop • Fenamiphos • Fenitrothion • Fenthion • Fosthiazate • GV • Isofluorophate • Isoxathion • Malaoxon • Malathion • Methamidophos • Methidathion • Metrifonate • Mevinphos • Monocrotophos • Naled • Novichok agent • Omethoate • Oxydemeton-Methyl • Paraoxon • Parathion • Parathion-Methyl • Phorate • Phosalone • Phosmet • Phostebupirim • Phoxim • Pirimiphos-Methyl • Sarin (GB) • Soman (GD) • Tabun (GA) • Temefos • Terbufos • Tetrachlorvinphos • Tribufos • Trichlorfon • VE • VG • VM • VR • VX; Others: Demecarium • Onchidal (Onchidella binneyi)BChE inhibitorsCymserine * Many of the acetylcholinesterase inhibitors listed above act as butyrylcholinesterase inhibitors.Others Choline (Lecithin) • Citicoline • Cyprodenate • Dimethylethanolamine (DMAE, deanol) • Glycerophosphocholine • Meclofenoxate (Centrophenoxine) • Phosphatidylcholine • Phosphatidylethanolamine • Phosphorylcholine • PirisudanolOthersAcetylcholine releasing agents: α-Latrotoxin • β-Bungarotoxin; Acetylcholine release inhibitors: Botulinum toxin (Botox); Acetylcholinesterase reactivators: Asoxime • Obidoxime • PralidoximeCategories:- Muscarinic agonists
- Mycotoxins
- Quaternary ammonium compounds
Wikimedia Foundation. 2010.
Look at other dictionaries:
MUSCARINE — Alcaloïde, de formule brute C9H212N, que l’on trouve dans divers champignons supérieurs comme l’amanite tue mouches (Amanita muscaria ), l’amanite panthère (Amanita pantherina ) et les inocybes. Ces champignons poussent dans les régions tempérées … Encyclopédie Universelle
Muscarine — Structure de la muscarine Général Nom IUPAC [(2S,4R,5S) 4 hydroxy 5 méthyltétrahydrofuran 2 yl] N,N,N triméthylm … Wikipédia en Français
muscarine — [mus′kə rin, mus′kərēn΄] n. [< ModL (Amanita) muscaria, fly (agaric) < L muscarius, of flies < musca, a fly: see MIDGE] an extremely poisonous alkaloid, C9H21NO3, found in certain mushrooms, rotten fish, etc., that will seriously disrupt … English World dictionary
muscarine — noun Etymology: German Muskarin, from New Latin (Amanita) muscaria fly agaric Date: 1872 a toxic alkaloid base [C9H20NO2]+ that is biochemically related to acetylcholine, is found especially in fly agaric, and acts directly on smooth muscle … New Collegiate Dictionary
muscarine — Toxin (alkaloid) from the mushroom Amanita muscaria (Fly Agaric) that binds to (muscarinic) acetylcholine receptors … Dictionary of molecular biology
muscarine — /mus keuhr in, keuh reen /, n. Chem. a poisonous compound, C8H19NO3, found in certain mushrooms, esp. fly agaric, and in decaying fish. [1870 75; < L muscar(ius) of flies (musc(a) fly + arius ARY) + INE1] * * * … Universalium
muscarine — noun An extremely poisonous alkaloid, obtained from fly agaric, that disrupts the action of acetylcholine neurotransmitter … Wiktionary
muscarine — A toxin with neurologic effects, first isolated from Amanita muscaria (fly agaric) and also present in some species of Hebeloma and Inocybe. The quaternary trimethylammonium salt of 2 methyl 3 hydroxy 5 (aminomethyl)tetrahydrofuran, it is a… … Medical dictionary
muscarine — (entrée créée par le supplément) (mu ska ri n ) s. f. Terme de chimie. Alcaloïde trouvé dans l ammanita muscaria ou fausse oronge ; il est très vénéneux … Dictionnaire de la Langue Française d'Émile Littré
muscarine — mÊŒskÉ™rɪËn , rɪn n. poisonous compound, alkaloid found in some kinds of mushrooms (Chemistry) … English contemporary dictionary