- Phosphatidylcholine
-
Phosphatidylcholines (PC) are a class of phospholipids that incorporate choline as a headgroup. They are a major component of biological membranes and can be easily obtained from a variety of readily available sources such as egg yolk or soy beans from which they are mechanically extracted or chemically extracted using hexane. They are also a member of the lecithin group of yellow-brownish fatty substances occurring in animal and plant tissues.
The name "lecithin" was originally defined from the Greek lekithos (λεκιθος, egg yolk) by Theodore Nicolas Gobley, a French chemist and pharmacist of the mid-19th century, who applied it to the egg yolk phosphatidylcholine that he identified in 1847 and finally completely described from a chemical structural point of view in 1874. Phosphatidylcholines are such a major component of lecithin that in some contexts the terms are sometimes used as synonyms. However, lecithin extract consists of a mixture of phosphatidylcholine and other compounds. It is also used along with sodium taurocholate for simulating fed- and fasted-state biorelevant media in dissolution studies of highly-lipophilic drugs.
Contents
Function
Phosphatidylcholine is a major constituent of cell membranes. Phosphatidylcholine is more commonly found in the exoplasmic or outer leaflet of a cell membrane. It is thought to be transported between membranes within the cell by phosphatidylcholine transfer protein (PCTP).[1]
Phosphatidylcholine also plays a role in membrane-mediated cell signalling and PCTP activation of other enzymes.[2]
Structure
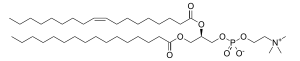
The phospholipid is composed of a choline head group and glycerophosphoric acid with a variety of fatty acids, one being a saturated fatty acid (in the example, here palmitic or hexadecanoic acid, H3C-(CH2)14-COOH; margaric acid identified by Gobley in egg yolk, or heptadecanoic acid H3C-(CH2)15-COOH, also belong to that class); and one being an unsaturated fatty acid (here oleic acid, or 9Z-octadecenoic acid, as in Gobley's original egg yolk lecithin).
Phospholipase D catalyzes the hydrolysis of phosphatidylcholine to form phosphatidic acid (PA), releasing the soluble choline headgroup into the cytosol.
Possible health benefits
Senescence
Phosphatidylcholine is a vital substance that is in every cell in the human body. Thus some researchers have used mutant mouse models with severe oxidative damage as a model of "accelerated aging" to investigate the possible role of phosphatidylcholine supplementation as a way of slowing down aging-related processes. [3] and improving brain functioning and memory capacity in dementia.[4] However, a systematic review of clinical trials in humans found that lecithin or phosphatidylcholine supplementation does not benefit patients with dementia.[5]
Liver repair
Recent studies point to the many potential benefits of phosphatidylcholine for liver repair. One study shows phosphatidylcholine's healing effect with hepatitis A, hepatitis B, and hepatitis C. Phosphatidylcholine administration for chronic, active hepatitis resulted in significant reduction of disease activity.[6]
Lipolysis
Some organizations promote the use of injected phosphatidylcholine, otherwise known as injection lipolysis, claiming the procedure can break down fat cells, and thus serve as an alternative to liposuction. It is important to note that while the procedure cites early experiments that showed lipolysis in cases of fat emboli,[7] no peer-reviewed studies have shown any amount of lipolysis even remotely comparable to liposuction.[8][9]
Ulcerative colitis
Phase IIa/b clinical trials performed at the Heidelberg University Hospital[10] have shown that delayed release phosphatidylcholine is an anti-inflammatory, and secondly, is a surface hydrophobicity increasing compound with promising therapeutic potential in the treatment of inflammatory bowel disease.
Possible health risks
In addition to the increased caloric burden of a diet rich in fats like phosphatidylcholine, a recent report has linked the microbial catabolites of phosphatidylcholine with increased atherosclerosis through the production of choline, trimethylamine oxide, and betaine.[11]
See also
- CDP choline
- Lysophosphatidylcholine
- Theodore Nicolas Gobley, discoverer of egg yolk lecithin, the first in history phosphatidylcholine
- Saturated fatty acid
- Unsaturated fatty acid
Additional images
References
- ^ Wirtz KW (July 1991). "Phospholipid transfer proteins.". Ann. Rev. Biochem. 60 (13): 73–99. doi:10.1146/annurev.bi.60.070191.000445. PMID 1883207.
- ^ Kanno K, Wu MK, Agate DA, Fanelli BK, Wagle N, Scapa EF, Ukomadu C, Cohen DE (October 2007). "Interacting proteins dictate function of the minimal START domain phosphatidylcholine transfer protein/StarD2.". J. Biol. Chem. 282 (42): 30728–36. doi:10.1074/jbc.M703745200. PMID 17704541.
- ^ Mei-Chu Hung, Koji Shibasaki, Riki Yoshida, Masao Sato and Katsumi Imaizumi (2001). Learning behaviour and cerebral protein kinase C, antioxidant status, lipid composition in senescence-accelerated mouse: influence of a phosphatidylcholine–vitamin B12 diet. British Journal of Nutrition, 86, pp 163-171 doi:10.1079/BJN2001391
- ^ Chung, Shu-Ying, Tomoe Moriyama, Eiko Uezu, Kayoko Uezu, Rieko Hirata, Noriko Yohena, Yasnunobu Masuda, Toyohiko Kokubu, and Shigeru Yamamoto. 1995. "Administration of Phosphatidylcholine Increases Brain Acetylcholine Concentration and Improves Memory in Mice with Dementia." The Journal of Nutrition 125: 1484-489. Print.
- ^ Higgins, Julian; Leon Flicker (21 JAN 2009). "Lecithin for dementia and cognitive impairment.". The Cochrane Library of Systematic Reviews 4: CD001015. PMID 12917896 doi: 10.1002/14651858.. http://onlinelibrary.wiley.com/doi/10.1002/14651858.CD001015/abstract. Retrieved 2011-11-19.
- ^ Tandy S, Phosphatidylcholine Supplementation Reduces Hepatic Lipid Levels in Mice. Nutrition and Metabolism Group, Heart Research Institute, Australia. (2010)
- ^ Hasegawa, Toshio; Matsukura, Tomoyuki; Ikeda, Shigaku (2009). "Mesotherapy for Benign Symmetric Lipomatosis". Aesthetic Plastic Surgery 34 (2): 153–156. doi:10.1007/s00266-009-9374-4. PMID 19488808. | title = Mesotherapy for Benign Symmetric Lipomatosis | journal = Aesthetic Plastic Surgery | year = 2010 | first = Toshio | last = Hasegawa | coauthors = Tomoyuki Matsukura and Shigaku Ikeda | volume = 34 | issue = 2 | pages = 153–156| pmid = 19488808 doi: 10.1007/s00266-009-9374-4 | url = http://www.springerlink.com/content/pn55k77954un9217/ | accessdate = 2010-08-23 | quote = Intralesional injection, termed mesotherapy, using phosphatidylcholine is a potentially effective therapy for benign symmetric lipomatosis that should be reconsidered as a therapeutic option for this disease. }}
- ^ Rotunda, Adam M.; Kolodney, Michael S. (2006). "Mesotherapy and Phosphatidylcholine Injections: Historical Clarification and Review". Dermatologic Surgery 32 (4): 465–480. doi:10.1111/j.1524-4725.2006.32100.x. PMID 16681654. | title = Mesotherapy and Phosphatidylcholine Injections: Historical Clarification and Review | journal = Dermatologic Surgery | date = 24 April 2006 | first = Adam M. | last = Rotunda | coauthors = Michael S. Kolodney | volume = 32 | issue = 4 | pages = 465–480| pmid = 16681654 Template:Doi =10.1111/j.1524-4725.2006.32100.x | url = http://onlinelibrary.wiley.com/doi/10.1111/j.1524-4725.2006.32100.x/abstract | accessdate = 2010-08-23 | quote = Recent laboratory investigations17 demonstrate that sodium deoxycholate, a bile salt also used as a laboratory detergent,102,103 was just as potent at causing adipocyte lysis and cell death as the complete phosphatidylcholine formula, which contains both phosphatidylcholine and deoxycholate (Figure 3). This bile salt is used to solubilize phosphatidylcholine by forming mixed micelles composed of phosphatidylcholine and deoxycholate.102,104 It is common practice to combine intravenous medications with bile salts to improve their water solubility.105,106 These findings suggest that sodium deoxycholate is the primary active ingredient in the phosphatidylchloline preparations.}}
- ^ Park, Seung Ha; Kim, Deok Woo; Lee, Min Ah; Yoo, Sang Chul; Rhee, Seung Chul; Koo, Sang Hwan; Seol, Geun Hye; Cho, Eun Young (2008). "Effectiveness of Mesotherapy on Body Contouring". Plastic and Reconstructive Surgery 121 (4): 179e–185e. doi:10.1097/01.prs.0000304611.71480.0a. PMID 18349597. | title = Effectiveness of Mesotherapy on Body Contouring | journal = Plastic & Reconstructive Surgery | date = april 2008 | first = Seung Ha | last = Park M.D. | coauthors = Kim, Deok Woo M.D.; Lee, Min Ah M.D.; Yoo, Sang Chul M.D.; Rhee, Seung Chul M.D.; Koo, Sang Hwan M.D., Ph.D.; Seol, Geun Hye R.N.; Cho, Eun Young A.N | volume = 121 | issue = 4 | pages = 179e-185e| pmid = 18349597 doi: 10.1097/01.prs.0000304611.71480.0a | accessdate = 2010-08-23 | quote = the author, when discussing phosphatidylcholine as a part of mesotherapy concludes --Although there is a preliminary report contradictory to this result,13 there was no body contouring observed in this study. There were no statistically significant changes in thigh girth, cross-sectional area, or laboratory values for the lipid profile except for a decrease in the triglyceride level in the blood, which might be an indirect effect of the method of aminophylline absorption into the systemic circulation}}
- ^ Schneider, H.; Braun, A.; Füllekrug, J.; Stremmel, W.; Ehehalt, R. (2010). "Lipid Based Therapy for Ulcerative Colitis—Modulation of Intestinal Mucus Membrane Phospholipids as a Tool to Influence Inflammation". International Journal of Molecular Sciences 11 (10): 4149. doi:10.3390/ijms11104149. PMC 2996791. PMID 21152327. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2996791.
- ^ Wang Z et al. (April 2011). "Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease". Nature 472 (7341): 57–63. doi:10.1038/nature09922. PMC 3086762. PMID 21475195. http://www.nature.com/nature/journal/v472/n7341/full/nature09922.html.
External links
- MeSH Phosphatidylcholines
- http://www.kvue.com/news/top/stories/110507kvuelipodissolve-mm.1e0189bdb.html
Glycerol backbone
(Glycerophospholipids/
Phosphoglycerides)Phosphatidyl-: -ethanolamine/cephalin (PE) · -choline/lechithin (PC) · -serine (PS) · -glycerol (PG) · -inositol (PI) (glyco- (GPI))
Phosphoinositides: PIP (PI(3)P, PI(4)P, PI(5)P) · PIP2 (PI(3,4)P2, PI(3,5)P2, PI(4,5)P2) · PIP3
Ether lipids: Plasmalogen (Platelet-activating factor)Sphingosine backbone Metabolites biochemical families: prot · nucl · carb (glpr, alco, glys) · lipd (fata/i, phld, strd, gllp, eico) · amac/i · ncbs/i · ttpy/iCell signaling: lipid signaling Intracellular CytosolSarcoplasmic reticulumOtherExtracellular lipid ligands Nootropics (N06B) Acetylcholinesterases Ampakines CX-516 • CX-546 • CX-614 • CX-691 • CX-717 • IDRA-21 • LY-404,187 • LY-503,430 • PEPA • Sunifiram • UnifiramD1 Agonists Eugeroics GABAA α5 Inverse Agonists H3 Antagonists mACh Agonists Alvameline • Arecoline • Cevimeline • CI-1017 • Milameline • Sabcomeline • Talsaclidine • Tazomeline • XanomelinenACh Agonists Racetams Others Acetylcarnitine • Adafenoxate • Bifemelane • Bilobalide (Ginkgo Biloba) • Carbenoxolone • Cerlapirdine • Choline (Lecithin) • Citicoline • Cyprodenate • Dimethylethanolamine • Ensaculin • Fipexide • Idebenone • Indeloxazine • Latrepirdine • Leteprinim • Linopirdine • Meclofenoxate • Nizofenone • Pirisudanol • Pyritinol • S-17092 • Sulbutiamine • Taltirelin • Teniloxazine • Tricyanoaminopropene • VinpocetineCholinergics Receptor ligands Agonists: 77-LH-28-1 • AC-42 • AC-260,584 • Aceclidine • Acetylcholine • AF30 • AF150(S) • AF267B • AFDX-384 • Alvameline • AQRA-741 • Arecoline • Bethanechol • Butyrylcholine • Carbachol • CDD-0034 • CDD-0078 • CDD-0097 • CDD-0098 • CDD-0102 • Cevimeline • cis-Dioxolane • Ethoxysebacylcholine • LY-593,039 • L-689,660 • LY-2,033,298 • McNA343 • Methacholine • Milameline • Muscarine • NGX-267 • Ocvimeline • Oxotremorine • PD-151,832 • Pilocarpine • RS86 • Sabcomeline • SDZ 210-086 • Sebacylcholine • Suberylcholine • Talsaclidine • Tazomeline • Thiopilocarpine • Vedaclidine • VU-0029767 • VU-0090157 • VU-0152099 • VU-0152100 • VU-0238429 • WAY-132,983 • Xanomeline • YM-796
Antagonists: 3-Quinuclidinyl Benzilate • 4-DAMP • Aclidinium Bromide • Anisodamine • Anisodine • Atropine • Atropine Methonitrate • Benactyzine • Benzatropine (Benztropine) • Benzydamine • BIBN 99 • Biperiden • Bornaprine • CAR-226,086 • CAR-301,060 • CAR-302,196 • CAR-302,282 • CAR-302,368 • CAR-302,537 • CAR-302,668 • CS-27349 • Cyclobenzaprine • Cyclopentolate • Darifenacin • DAU-5884 • Dimethindene • Dexetimide • DIBD • Dicyclomine (Dicycloverine) • Ditran • EA-3167 • EA-3443 • EA-3580 • EA-3834 • Elemicin • Etanautine • Etybenzatropine (Ethylbenztropine) • Flavoxate • Himbacine • HL-031,120 • Ipratropium bromide • J-104,129 • Hyoscyamine • Mamba Toxin 3 • Mamba Toxin 7 • Mazaticol • Mebeverine • Methoctramine • Metixene • Myristicin • N-Ethyl-3-Piperidyl Benzilate • N-Methyl-3-Piperidyl Benzilate • Orphenadrine • Otenzepad • Oxybutynin • PBID • PD-102,807 • PD-0298029 • Phenglutarimide • Phenyltoloxamine • Pirenzepine • Piroheptine • Procyclidine • Profenamine • RU-47,213 • SCH-57,790 • SCH-72,788 • SCH-217,443 • Scopolamine (Hyoscine) • Solifenacin • Telenzepine • Tiotropium bromide • Tolterodine • Trihexyphenidyl • Tripitamine • Tropatepine • Tropicamide • WIN-2299 • Xanomeline • Zamifenacin; Others: 1st Generation Antihistamines (Brompheniramine, chlorphenamine, cyproheptadine, dimenhydrinate, diphenhydramine, doxylamine, mepyramine/pyrilamine, phenindamine, pheniramine, tripelennamine, triprolidine, etc) • Tricyclic Antidepressants (Amitriptyline, doxepin, trimipramine, etc) • Tetracyclic Antidepressants (Amoxapine, maprotiline, etc) • Typical Antipsychotics (Chlorpromazine, thioridazine, etc) • Atypical Antipsychotics (Clozapine, olanzapine, quetiapine, etc)Agonists: 5-HIAA • A-84,543 • A-366,833 • A-582,941 • A-867,744 • ABT-202 • ABT-418 • ABT-560 • ABT-894 • Acetylcholine • Altinicline • Anabasine • Anatoxin-a • AR-R17779 • Butyrylcholine • Carbachol • Cotinine • Cytisine • Decamethonium • Desformylflustrabromine • Dianicline • Dimethylphenylpiperazinium • Epibatidine • Epiboxidine • Ethanol • Ethoxysebacylcholine • EVP-4473 • EVP-6124 • Galantamine • GTS-21 • Ispronicline • Lobeline • MEM-63,908 (RG-3487) • Nicotine • NS-1738 • PHA-543,613 • PHA-709,829 • PNU-120,596 • PNU-282,987 • Pozanicline • Rivanicline • Sazetidine A • Sebacylcholine • SIB-1508Y • SIB-1553A • SSR-180,711 • Suberylcholine • TC-1698 • TC-1734 • TC-1827 • TC-2216 • TC-5214 • TC-5619 • TC-6683 • Tebanicline • Tropisetron • UB-165 • Varenicline • WAY-317,538 • XY-4083
Antagonists: 18-Methoxycoronaridine • α-Bungarotoxin • α-Conotoxin • Alcuronium • Amantadine • Anatruxonium • Atracurium • Bupropion (Amfebutamone) • Chandonium • Chlorisondamine • Cisatracurium • Coclaurine • Coronaridine • Dacuronium • Decamethonium • Dextromethorphan • Dextropropoxyphene • Dextrorphan • Diadonium • DHβE • Dimethyltubocurarine (Metocurine) • Dipyrandium • Dizocilpine (MK-801) • Doxacurium • Duador • Esketamine • Fazadinium • Gallamine • Hexafluronium • Hexamethonium (Benzohexonium) • Ibogaine • Isoflurane • Ketamine • Kynurenic acid • Laudexium (Laudolissin) • Levacetylmethadol • Malouetine • Mecamylamine • Memantine • Methadone • Methorphan (Racemethorphan) • Methyllycaconitine • Metocurine • Mivacurium • Morphanol (Racemorphanol) • Neramexane • Nitrous Oxide • Pancuronium • Pempidine • Pentamine • Pentolinium • Phencyclidine • Pipecuronium • Radafaxine • Rapacuronium • Rocuronium • Surugatoxin • Suxamethonium (Succinylcholine) • Thiocolchicoside • Toxiferine • Trimethaphan • Tropeinium • Tubocurarine • Vecuronium • XenonReuptake inhibitors PlasmalemmalCHT InhibitorsVAChT InhibitorsEnzyme inhibitors ChAT inhibitors1-(-Benzoylethyl)pyridinium • 2-(α-Naphthoyl)ethyltrimethylammonium • 3-Chloro-4-stillbazole • 4-(1-Naphthylvinyl)pyridine • Acetylseco hemicholinium-3 • Acryloylcholine • AF64A • B115 • BETA • CM-54,903 • CatabolismAChE inhibitorsReversible: Carbamates: Aldicarb • Bendiocarb • Bufencarb • Carbaryl • Carbendazim • Carbetamide • Carbofuran • Chlorbufam • Chloropropham • Ethienocarb • Ethiofencarb • Fenobucarb • Fenoxycarb • Formetanate • Furadan • Ladostigil • Methiocarb • Methomyl • Miotine • Oxamyl • Phenmedipham • Pinmicarb • Pirimicarb • Propamocarb • Propham • Propoxur; Stigmines: Ganstigmine • Neostigmine • Phenserine • Physostigmine • Pyridostigmine • Rivastigmine; Others: Acotiamide • Ambenonium • Donepezil • Edrophonium • Galantamine • Huperzine A • Minaprine • Tacrine • Zanapezil
Irreversible: Organophosphates: Acephate • Azinphos-methyl • Bensulide • Cadusafos • Chlorethoxyfos • Chlorfenvinphos • Chlorpyrifos • Chlorpyrifos-Methyl • Coumaphos • Cyclosarin (GF) • Demeton • Demeton-S-Methyl • Diazinon • Dichlorvos • Dicrotophos • Diisopropyl fluorophosphate (Guthion) • Diisopropylphosphate • Dimethoate • Dioxathion • Disulfoton • EA-3148 • Echothiophate • Ethion • Ethoprop • Fenamiphos • Fenitrothion • Fenthion • Fosthiazate • GV • Isofluorophate • Isoxathion • Malaoxon • Malathion • Methamidophos • Methidathion • Metrifonate • Mevinphos • Monocrotophos • Naled • Novichok agent • Omethoate • Oxydemeton-Methyl • Paraoxon • Parathion • Parathion-Methyl • Phorate • Phosalone • Phosmet • Phostebupirim • Phoxim • Pirimiphos-Methyl • Sarin (GB) • Soman (GD) • Tabun (GA) • Temefos • Terbufos • Tetrachlorvinphos • Tribufos • Trichlorfon • VE • VG • VM • VR • VX; Others: Demecarium • Onchidal (Onchidella binneyi)BChE inhibitorsCymserine * Many of the acetylcholinesterase inhibitors listed above act as butyrylcholinesterase inhibitors.Others Choline (Lecithin) • Citicoline • Cyprodenate • Dimethylethanolamine (DMAE, deanol) • Glycerophosphocholine • Meclofenoxate (Centrophenoxine) • Phosphatidylcholine • Phosphatidylethanolamine • Phosphorylcholine • PirisudanolOthersAcetylcholine releasing agents: α-Latrotoxin • β-Bungarotoxin; Acetylcholine release inhibitors: Botulinum toxin (Botox); Acetylcholinesterase reactivators: Asoxime • Obidoxime • PralidoximeCategories:- Phospholipids
Wikimedia Foundation. 2010.
Look at other dictionaries:
Phosphatidylcholine — Fig 1. 1 exemple de phosphatidylcholine, la palmitoyl oleyl sn phosphatidylcholine. En général, une lécithine comporte un acide gras saturé et acide gras insaturé. La phosphatidylcholine est plus connue sous le nom lécithine ; cette… … Wikipédia en Français
Phosphatidylcholine-retinol O-acyltransferase — In enzymology, a phosphatidylcholine retinol O acyltransferase (EC number|2.3.1.135) is an enzyme that catalyzes the chemical reaction:phosphatidylcholine + retinol [cellular retinol binding protein] ightleftharpoons 2 acylglycerophosphocholine + … Wikipedia
Phosphatidylcholine-dolichol O-acyltransferase — In enzymology, a phosphatidylcholine dolichol O acyltransferase (EC number|2.3.1.83) is an enzyme that catalyzes the chemical reaction:3 sn phosphatidylcholine + dolichol ightleftharpoons 1 acyl sn glycero 3 phosphocholine + acyldolicholThus, the … Wikipedia
Phosphatidylcholine-sterol O-acyltransferase — In enzymology, a phosphatidylcholine sterol O acyltransferase (EC number|2.3.1.43) is an enzyme that catalyzes the chemical reaction:phosphatidylcholine + a sterol ightleftharpoons 1 acylglycerophosphocholine + a sterol esterThus, the two… … Wikipedia
Phosphatidylcholine synthase — In enzymology, a phosphatidylcholine synthase (EC number|2.7.8.24) is an enzyme that catalyzes the chemical reaction:CDP diacylglycerol + choline ightleftharpoons CMP + phosphatidylcholineThus, the two substrates of this enzyme are CDP… … Wikipedia
Phosphatidylcholine desaturase — In enzymology, a phosphatidylcholine desaturase (EC number|1.3.1.35) is an enzyme that catalyzes the chemical reaction:1 acyl 2 oleoyl sn glycero 3 phosphocholine + NAD+ ightleftharpoons 1 acyl 2 linoleoyl sn glycero 3 phosphocholine + NADH +… … Wikipedia
Phosphatidylcholine 12-monooxygenase — In enzymology, a phosphatidylcholine 12 monooxygenase (EC number|1.14.13.26) is an enzyme that catalyzes the chemical reaction:1 acyl 2 oleoyl sn glycero 3 phosphocholine + NADH + H+ + O2 ightleftharpoons 1 acyl 2 [(S) 12 hydroxyoleoyl] sn… … Wikipedia
phosphatidylcholine — noun Date: 1954 lecithin … New Collegiate Dictionary
phosphatidylcholine — phos·pha·ti·dyl·cho·line (fŏs fə tīd l kōʹlēn ) n. See lecithin. [phosphate + ide + yl + choline.] * * * … Universalium
phosphatidylcholine — noun A phospholipid containing choline. Syn: lecithin … Wiktionary