- CX717
-
CX717 Clinical data Pregnancy cat. ? Legal status ? Routes oral, IV Identifiers CAS number 867276-98-0 
ATC code None Chemical data Formula ?  (what is this?)
(what is this?)CX717 is an ampakine compound created by Christopher Marrs and Gary Rogers in 1996[1] at Cortex Pharmaceuticals. It affects the neurotransmitter glutamate, with trials showing the drug improves cognitive functioning and memory.[2]
Contents
Approval process
In 2005 the U.S. Food and Drug Administration (FDA) accepted Cortex Pharmaceuticals' Investigational New Drug (IND) application to initiate pilot Phase II clinical trials in the United States.
Also, in 2005, the United States Department of Defense funded a study to look into CX717 and the physiological effects of sleepiness. The study found that rhesus monkeys performed faster and better after receiving the drug, and it counteracted the effects of sleep deprivation.
However, a 2006 study funded by DARPA found that CX717 did not improve cognitive performance in humans subjected to simulated night shift work.[3]
In early March 2006 Cortex reported that, in a small pilot Phase II study, CX717 had demonstrated positive clinical and statistical results on the primary endpoint, the ADHD rating scale and the sub-scales related to attention and hyperactivity which are used for the approval of all currently available ADHD treatments. According to a Cortex Pharmaceuticals press release, "Consistent with all previous studies involving over 220 patients and healthy adults, this study demonstrated that CX717 was safe, well tolerated, and produced no increase in heart rate, blood pressure or other cardiovascular side effects".
In April 2007 Cortex Pharmaceuticals submitted two large data packages to the FDA regarding CX717. One data set went to the FDA's Division of Neurology Drug Products for the treatment of Alzheimer's disease, while the other went to the Division of Psychiatry Products where the company filed a second CX717 IND for the treatment of ADHD. According to a Cortex Pharmaceuticals press release, the submitted data package "provides clear evidence that the specific histopathological changes seen in animal toxicology studies, which previously caused the FDA to put CX717 on clinical hold, is a postmortem fixation artifact and is not found in the tissue of the animal when it is still living".[4]
Roger G Stoll PhD, Chief Executive Officer of Cortex, stated,
“When CX717 was removed from clinical hold on October 6, 2006 by the Neurology Division a dose was permitted for continuing a study in patients with Alzheimer's disease, but that dose was too low to permit the assessment of the drug in patients with ADHD. Further information was needed to better understand the cause of the histopathological changes. We now have a substantial data base which clearly documents the fact that the histological changes of concern occur postmortem when the fixative solution is used to prepare the slides of the tissue specimens.”
However, in October 2007 the FDA denied Cortex's IND application for a Phase IIb study of CX717 for treatment of ADHD, based on the same animal toxicology results. Cortex responded by inactivating the application, although it will "continue its plans to develop CX717 for the acute treatment of respiratory depression (RD) and continue its study of CX717 in its Alzheimer’s disease PET scan study. Cortex believes that the IND application previously filed with the Division of Neurology Products of the FDA for the treatment of Alzheimer’s disease will not be affected by the actions of the DPP."[5] The company hopes that after the use of the compound in treating a high-risk acute condition is approved and well-established, the risks of longer-term use at higher doses, such as for treatment of ADHD, will be shown to be less than the FDA had concluded.
Use for reversal of respiratory depression
The relatively poor oral bioavailability and blood-brain barrier penetration of CX-717 ultimately led to Cortex abandoning development of the 800 mg oral formulation of CX-717 for ADHD,[6] although research into its action in the brain continues.[2] However the unexpected discovery of the strong respiratory stimulant effects of the ampakine drugs on the pre-Botzinger complex of the brain has led to continued development of an intravenous formulation of CX-717 for use alongside opioid analgesics,[7] along with an oral formulation of CX-1739, which is around 3-5x more potent than CX-717 and has better oral bioavailability, and is being trialled for treatment of sleep apnoea.[8] Further research has investigated the neurological mechanisms behind the anti-respiratory depressant effects of CX-717,[9] and demonstrated that it can be used in humans alongside opioid drugs to reduce this side effect without affecting analgesia.[10]
Related AMPAkines
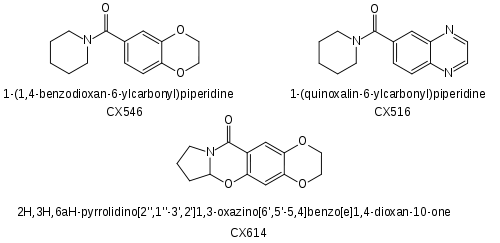
Other AMPAkine drugs from Cortex Pharmaceuticals such as CX-546 and CX-614 have already been researched for use in treating Alzheimer's disease and ADHD. These drugs were reasonably effective at reducing the symptoms of Alzheimer's and it was hoped that they could also slow the progression of the disease, but both CX-546 and CX-614 have poor bioavailability, and are only active at very high doses of 1000 mg or more. CX-717 and CX-1739 are newer and more potent drugs in the same series. The chemical structures of CX-717 and CX-1739 have not yet been revealed by Cortex Pharmaceuticals, but are presumably similar to earlier compounds in the series as shown below.[11][12][13] It is very unusual for research on a compound to be released in scientific journals without disclosing exactly what the compound consists of, but this information is likely to have been kept confidential for reasons of intellectual property, and also because the research on CX-717 was initially partially funded by DARPA, the United States Defense Advanced Research Projects Agency.
References
- ^ "Benzofurazan compounds for enhancing glutamatergic synaptic responses". http://www.patentstorm.us/patents/6110935-description.html. Retrieved 2008-04-04.
- ^ a b Hampson RE, España RA, Rogers GA, Porrino LJ, Deadwyler SA (January 2009). "Mechanisms underlying cognitive enhancement and reversal of cognitive deficits in nonhuman primates by the ampakine CX717". Psychopharmacology 202 (1-3): 355–69. doi:10.1007/s00213-008-1360-z. PMID 18985324.
- ^ "Cortex News & Events". Archived from the original on 2007-09-27. http://web.archive.org/web/20070927230732/http://www.cortexpharm.com/html/news/06/06-21-06.html. Retrieved 2007-10-21.
- ^ "Cortex's AMPAKINE CX-717 Toxicology Data Package Submitted to FDA". http://www.cortexpharm.com/news/07/txt_041807.html. Retrieved 2009-03-31.
- ^ "FDA’s Psychiatric Division has Rejected Cortex’s Request to Study CX717 in Phase IIb ADHD Study". http://www.businesswire.com/portal/site/home/permalink/?ndmViewId=news_view&newsId=20071011005369&newsLang=en. Retrieved 2008-02-28.
- ^ [1]
- ^ Ren J, Ding X, Funk GD, Greer JJ (June 2009). "Ampakine CX717 protects against fentanyl-induced respiratory depression and lethal apnea in rats". Anesthesiology 110 (6): 1364–70. doi:10.1097/ALN.0b013e31819faa2a. PMID 19461299.
- ^ Cortex Pharmaceuticals Press Release 1 June 2009.
- ^ Lorier AR, Funk GD, Greer JJ (2010). Hochman, Shawn. ed. "Opiate-induced suppression of rat hypoglossal motoneuron activity and its reversal by ampakine therapy". PLoS ONE 5 (1): e8766. doi:10.1371/journal.pone.0008766. PMC 2808240. PMID 20098731. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2808240.
- ^ Oertel BG, Felden L, Tran PV, Bradshaw MH, Angst MS, Schmidt H, Johnson S, Greer JJ, Geisslinger G, Varney MA, Lötsch J (February 2010). "Selective antagonism of opioid-induced ventilatory depression by an ampakine molecule in humans without loss of opioid analgesia". Clinical Pharmacology and Therapeutics 87 (2): 204–11. doi:10.1038/clpt.2009.194. PMID 19907420.
- ^ Mueller, R.; Li, Y. X.; Hampson, A.; Zhong, S.; Harris, C.; Marrs, C.; Rachwal, S.; Ulas, J. et al. (2011). "Benzoxazinones as potent positive allosteric AMPA receptor modulators: Part I". Bioorganic & Medicinal Chemistry Letters 21 (13): 3923. doi:10.1016/j.bmcl.2011.05.026.
- ^ Mueller, R.; Rachwal, S.; Tedder, M. E.; Li, Y. X.; Zhong, S.; Hampson, A.; Ulas, J.; Varney, M. et al. (2011). "Substituted benzoxazinones as potent positive allosteric AMPA receptor modulators: Part II". Bioorganic & Medicinal Chemistry Letters 21 (13): 3927. doi:10.1016/j.bmcl.2011.05.024.
- ^ Mueller, R.; Rachwal, S.; Lee, S.; Zhong, S.; Li, Y. X.; Haroldsen, P.; Herbst, T.; Tanimura, S. et al. (2011). "Benzotriazinone and benzopyrimidinone derivatives as potent positive allosteric AMPA receptor modulators". Bioorganic & Medicinal Chemistry Letters 21 (20): 6170. doi:10.1016/j.bmcl.2011.07.098.
- Bast T, da Silva BM, Morris RG (2005). "Distinct contributions of hippocampal NMDA and AMPA receptors to encoding and retrieval of one-trial place memory". J. Neurosci. 25 (25): 5845–56. doi:10.1523/JNEUROSCI.0698-05.2005. PMID 15976073.
- Arai AC, Kessler M, Rogers G, Lynch G (2000). "Effects of the potent ampakine CX614 on hippocampal and recombinant AMPA receptors: interactions with cyclothiazide and GYKI 52466". Mol. Pharmacol. 58 (4): 802–13. PMID 10999951. http://nootropics.com/ampakines/cx614.html.
- Porrino LJ, Daunais JB, Rogers GA, Hampson RE, Deadwyler SA (2005). "Facilitation of task performance and removal of the effects of sleep deprivation by an ampakine (CX717) in nonhuman primates". PLoS Biol. 3 (9): e299. doi:10.1371/journal.pbio.0030299. PMC 1188239. PMID 16104830. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=1188239.
- [2] Cortex Pharmaceuticals press release
See also
- Adrafinil
- AMPA
- Arecoline
- Carphedon
- CX-516 (Ampalex)
- CX-546
- CX-614
- Desmopressin
- Farampator
- Idebenone
- Modafinil
- Vasopressin
- Vinpocetine
Psychostimulants, agents used for ADHD, and nootropics (N06B) Centrally acting sympathomimetics Xanthine derivatives Glutamate receptor CX-516 • CX-546 • CX-614 • CX-691 • CX-717 • IDRA-21 • LY-404,187 • LY-503,430 • PEPA • S-18986 • Sunifiram • UnifiramEugeroics / Benzhydryl compounds Histamine H3 receptor antagonists GABAA α5 inverse agonists Dopamine D1 receptor agonists α7 nicotinic agonists / PAMs AR-R17779 • PNU-282,987 • SSR-180,711Prolyl endopeptidase inhibitors S-17092Alpha-adrenergic agonists Other psychostimulants and nootropics Acetylcarnitine • Adafenoxate • Bifemelane • Carbenoxolone • Citicoline • Cyprodenate • Ensaculin • Idebenone • Ispronicline • Deanol • Dimebon • Fipexide • Leteprinim • Linopirdine • Meclofenoxate • Nizofenone • P7C3 • Pirisudanol • Pyritinol • Rubidium • Sulbutiamine • Taltirelin • Tricyanoaminopropene • VinpocetineGlutamatergics Ionotropic Agonists: 5-Fluorowillardiine • AMPA • Domoic acid • Quisqualic acid; Positive allosteric modulators: Aniracetam • Cyclothiazide • CX-516 • CX-546 • CX-614 • CX-691 • CX-717 • Diazoxide • HCTZ • IDRA-21 • LY-392,098 • LY-404,187 • LY-451,395 • LY-451,646 • LY-503,430 • Oxiracetam • PEPA • Piracetam • Pramiracetam • S-18986 • Sunifiram • Unifiram
Antagonists: ATPO • Barbiturates • Caroverine • CNQX • DNQX • GYKI-52466 • NBQX • Perampanel • Talampanel • Tezampanel • Topiramate; Negative allosteric modulators: GYKI-53,655Agonists: Glutamate/acite site competitive agonists: Aspartate • Glutamate • Homoquinolinic acid • Ibotenic acid • NMDA • Quinolinic acid • Tetrazolylglycine; Glycine site agonists: ACBD • ACPC • ACPD • Alanine • CCG • Cycloserine • DHPG • Fluoroalanine • Glycine • HA-966 • L-687,414 • Milacemide • Sarcosine • Serine • Tetrazolylglycine; Polyamine site agonists: Acamprosate • Spermidine • Spermine
Antagonists: Competitive antagonists: AP5 (APV) • AP7 • CGP-37849 • CGP-39551 • CGP-39653 • CGP-40116 • CGS-19755 • CPP • LY-233,053 • LY-235,959 • LY-274,614 • MDL-100,453 • Midafotel (d-CPPene) • NPC-12,626 • NPC-17,742 • PBPD • PEAQX • Perzinfotel • PPDA • SDZ-220581 • Selfotel; Noncompetitive antagonists: ARR-15,896 • Caroverine • Dexanabinol • FPL-12495 • FR-115,427 • Hodgkinsine • Magnesium • MDL-27,266 • NPS-1506 • Psychotridine • Zinc; Uncompetitive pore blockers: 2-MDP • 3-MeO-PCP • 8A-PDHQ • Alaproclate • Amantadine • Aptiganel • ARL-12,495 • ARL-15,896-AR • ARL-16,247 • Budipine • Delucemine • Dexoxadrol • Dextrallorphan • Dieticyclidine • Dizocilpine • Endopsychosin • Esketamine • Etoxadrol • Eticyclidine • Gacyclidine • Ibogaine • Indantadol • Ketamine • Ketobemidone • Loperamide • Memantine • Meperidine (Pethidine) • Methadone • Methorphan (Dextromethorphan, Levomethorphan) • Methoxetamine • Milnacipran • Morphanol (Dextrorphan, Levorphanol) • NEFA • Neramexane • Nitrous oxide • Noribogaine • Orphenadrine • PCPr • Phencyclamine • Phencyclidine • Propoxyphene • Remacemide • Rhynchophylline • Riluzole • Rimantadine • Rolicyclidine • Sabeluzole • Tenocyclidine • Tiletamine • Tramadol • Xenon; Glycine site antagonists: ACEA-1021 • ACEA-1328 • ACPC • Carisoprodol • CGP-39653 • CKA • DCKA • Felbamate • Gavestinel • GV-196,771 • Kynurenic acid • L-689,560 • L-701,324 • Lacosamide • Licostinel • LU-73,068 • MDL-105,519 • Meprobamate • MRZ 2/576 • PNQX • ZD-9379; NR2B subunit antagonists: Besonprodil • CO-101,244 (PD-174,494) • CP-101,606 • Eliprodil • Haloperidol • Ifenprodil • Isoxsuprine • Nylidrin • Ro8-4304 • Ro25-6981 • Traxoprodil; Polyamine site antagonists: Arcaine • Co 101676 • Diaminopropane • Acamprosate • Diethylenetriamine • Huperzine A • Putrescine • Ro 25-6981; Unclassified/unsorted antagonists: Chloroform • Diethyl ether • Enflurane • Ethanol (Alcohol) • Halothane • Isoflurane • Methoxyflurane • Toluene • Trichloroethane • Trichloroethanol • Trichloroethylene • XyleneAgonists: 5-Iodowillardiine • ATPA • Domoic acid • Kainic acid • LY-339,434 • SYM-2081
Antagonists: CNQX • DNQX • LY-382,884 • NBQX • NS102 • Tezampanel • Topiramate • UBP-302; Negative allosteric modulators: NS-3763Metabotropic Agonists: Unselective: ACPD • DHPG • Quisqualic acid; mGlu1-selective: Ro01-6128 • Ro67-4853 • Ro67-7476 • VU-71; mGlu5-selective: ADX-47273 • CDPPB • CHPG • DFB • VU-1545
Antagonists: Unselective: MCPG • NPS-2390; mGlu1-selective: BAY 36-7620 • CPCCOEt • LY-367,385 • LY-456,236; mGlu5-selective: Dipraglurant • DMeOB • Fenobam • LY-344,545 • MPEP • MTEP • SIB-1757 • SIB-1893Agonists: Unselective: L-AP4; mGlu4-selective: PHCCC • VU-001,171 • VU-0155,041; mGlu7-selective: AMN082; mGlu8-selective: DCPG
Antagonists: Unselective: CPPG • MAP4 • MSOP • MPPG • MTPG • UBP-1112; mGlu7-selective: MMPIPTransporter
inhibitorsDHKA • PDC • WAY-213,613vGluTs7-CKA • Evans blueCategories:- Ampakines
- Stimulants
Wikimedia Foundation. 2010.