- Loperamide
-
"Imodium" redirects here. For the song by Nirvana, see Breed (song).
Loperamide 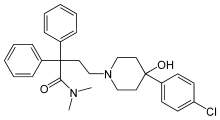
Systematic (IUPAC) name 4-[4-(4-chlorophenyl)-4-hydroxypiperidin-1-yl]- N,N-dimethyl-2,2-diphenylbutanamide Clinical data Trade names Imodium AHFS/Drugs.com monograph MedlinePlus a682280 Pregnancy cat. B[1] Legal status ? (CA) GSL (UK) OTC (US) Routes oral, insufflation Pharmacokinetic data Bioavailability Not significantly absorbed from the gut Protein binding 97% Metabolism hepatic Half-life 9.1 to 14.4 hours (average 10.8 hours) Identifiers CAS number 53179-11-6  34552-83-5 (with HCl)
34552-83-5 (with HCl)ATC code A07DA03 A07DA05 PubChem CID 3955 DrugBank DB00836 ChemSpider 3818 
UNII 6X9OC3H4II 
KEGG D08144 
ChEBI CHEBI:6532 
ChEMBL CHEMBL841 
Chemical data Formula C29H33ClN2O2 Mol. mass 477.037 g/mol (513.506 with HCl) SMILES eMolecules & PubChem  (what is this?) (verify)
(what is this?) (verify)Loperamide (
 /loʊˈpɛrəmaɪd/; R-18553[clarification needed]), a synthetic piperidine derivative,[2] is an opioid drug used against diarrhea resulting from gastroenteritis or inflammatory bowel disease. In most countries it is available generically and under brand names such as Lopex, Imodium, Dimor, Fortasec, and Pepto Diarrhea Control. It was developed at Janssen Pharmaceutica.[3]
/loʊˈpɛrəmaɪd/; R-18553[clarification needed]), a synthetic piperidine derivative,[2] is an opioid drug used against diarrhea resulting from gastroenteritis or inflammatory bowel disease. In most countries it is available generically and under brand names such as Lopex, Imodium, Dimor, Fortasec, and Pepto Diarrhea Control. It was developed at Janssen Pharmaceutica.[3]Contents
Medical uses
Loperamide is effective for the treatment of a number of types of diarrhea.[4]
Mechanism of action
Loperamide is an opioid-receptor agonist and acts on the μ-opioid receptors in the myenteric plexus of the large intestine; by itself it does not affect the central nervous system like other opioids, unless extremely high doses are taken (in which case it can produce psychoactive effects on its own).
It works by decreasing the activity of the myenteric plexus, which, like morphine, decreases the tone of the longitudinal smooth muscles but increases the tone of circular smooth muscles of the intestinal wall. This increases the amount of time substances stay in the intestine, allowing for more water to be absorbed out of the fecal matter. Loperamide also decreases colonic mass movements and suppresses the gastrocolic reflex.[5]
Many physicians and pharmacists believe that loperamide does not cross the blood–brain barrier. In fact, however, loperamide does cross this barrier, although it is immediately pumped back out into non–central nervous system (CNS) circulation by P-glycoprotein. While this mechanism effectively shields the CNS from exposure (and thus risk of CNS addiction) to loperamide, many drugs are known to inhibit P-glycoprotein and may thus render the CNS vulnerable to opiate agonism by loperamide.[6]
Only when very high doses are taken does enough accumulate in the brain to produce typical opioid effects, lasting for a number of hours (just as typical opioids would). Tolerance in response to long-term use has not been reported.
However, loperamide has been shown to cause a mild physical dependence during preclinical studies, specifically in mice, rats, and rhesus monkeys. Symptoms of mild opiate withdrawal have been observed following abrupt discontinuation of long-term therapy with loperamide.[7][8]
Contraindications
The use of Loperamide (Imodium) in children under 2 years is not recommended. There have been rare reports of fatal paralytic ileus associated with abdominal distention. Most of these reports occurred in the setting of acute dysentery, overdose, and with very young children less than two years of age.[9] In 1990, all pediatric formulations of the antidiarrheal loperamide (Imodium and others) were banned in Pakistan.[10]
Treatment should be avoided in the presence of fever or if the stool is bloody (dysentery).[11] It is of no value in diarrhea caused by cholera, Shigella or Campylobacter.[11] Treatment is not recommended for patients that could suffer detrimental effects from rebound constipation. If there is a suspicion of diarrhea associated with organisms that can penetrate the intestinal walls, such as E. coli O157:H7 or salmonella, loperamide is contraindicated.
Loperamide treatment is not used in symptomatic C. difficile infections, as it increases the risk of toxin retention and precipitation of toxic megacolon.
Adverse effects
Adverse drug reactions (ADRs) associated with Loperamide include abdominal pain and bloating, nausea, vomiting and constipation. Rare side-effects associated with Loperamide are paralytic ileus, dizziness and rashes.[12]
Hepatic
It is not recommended for use in hepatic failure since it can precipitate hepatic encephalopathy. [12]
Marketing in the developing world
In Pakistan, seven infants died in 1989 and 1990 after parents treated their babies' diarrhea with Imodium, a medicine sold by a subsidiary of Johnson & Johnson.
Since 1980, the World Health Organization has warned Third World doctors not to use Imodium because it can paralyze a child's intestines. For months a local doctor begged the company to pull the product from stores, but it didn't do so until a British television program broadcast footage of dying babies.[13]
Crossing the blood-brain barrier
Concurrent administration of P-glycoprotein inhibitors such as quinidine and its other isomer quinine (although much higher doses must be used), PPIs like omeprazole (Prilosec OTC) and even black pepper (piperine as the active ingredient) could potentially allow loperamide to cross the blood-brain barrier. It should however be noted that only quinidine with loperamide was found to produce respiratory depression, indicative of central opioid action.[14]
See also
- Simethicone - an anti-gas frequently combined with Loperamide in medications
- Gastroenteritis
- Traveler's diarrhea
- Methylnaltrexone - a Mu opioid antagonist (in contrast to Loperamide as an Mu opioid agonist) that doesn't cross the blood brain barrier so as to not affect CNS
References
- ^ "Loperamide Hydrochloride." DailyMed.
- ^ US National Cancer Institute, Drug Dictionary
- ^ R. A. Stokbroekx, J. Vanenberk, A. H. M. T. Van Heertum, G. M. L. W. van Laar, M. J. M. C. Van der Aa, W. F. M. Van Bever and P. A. J. Janssen (1973). "Synthetic Antidiarrheal Agents. 2,2-Diphenyl-4-(4'-aryl-4'-hydroxypiperidino)butyramides". J. Med. Chem., 16 (7): 782–786. doi:10.1021/jm00265a009.
- ^ Hanauer, SB (2008 Winter). "The role of loperamide in gastrointestinal disorders". Reviews in gastroenterological disorders 8 (1): 15–20. PMID 18477966.
- ^ Katzung, Bertram G. Basic and Clinical Pharmacology, 9th ed. (2004). ISBN 0-07-141092-9[page needed]
- ^ Clin Exp Pharmacol Physiol 2007;34:695–701.
- ^ Yanagita T, Miyasato K, Sato J (1979). "Dependence potential of loperamide studied in rhesus monkeys". NIDA Research Monograph 27: 106–13. PMID 121326.
- ^ Nakamura H, Ishii K, Yokoyama Y, et al. (November 1982). "[Physical dependence on loperamide hydrochloride in mice and rats]" (in Japanese). Yakugaku Zasshi 102 (11): 1074–85. PMID 6892112.
- ^ http://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?id=6651
- ^ http://www.essentialdrugs.org/edrug/archive/199708/msg00056.php
- ^ a b Butler T (October 2008). "Loperamide for the treatment of traveler's diarrhea: broad or narrow usefulness?". Clinical Infectious Diseases 47 (8): 1015–6. doi:10.1086/591704. PMID 18781871.
- ^ a b Australian Medicine Handbook. Bichner F, editor. Adelaide: Australian Medicine Handbook Pty Ltd; 2010.
- ^ Scanlan, Christopher (1991-06-09). "America's Deadly Exports -- Trade Of Toxic Products Abroad Is A Windfall For U.S. Companies". The Seattle Times. http://community.seattletimes.nwsource.com/archive/?date=19910609&slug=1288069.
- ^ Sadeque AJ, Wandel C, He H, Shah S, Wood AJ (September 2000). "Increased drug delivery to the brain by P-glycoprotein inhibition". Clinical Pharmacology and Therapeutics 68 (3): 231–7. doi:10.1067/mcp.2000.109156. PMID 11014404.
External links
Opioids Opium and
poppy straw
derivativesCrude opiate extracts/
whole opium productsCompote/Kompot/Polish heroin • Diascordium • B & O Supprettes • Dover's powder • Kendal Black Drop • Laudanum • Mithridate • Opium • Paregoric • Poppy straw concentrate • Poppy tea • Smoking opium • Theriac
Natural opiates Opium alkaloids
see also: Components of opiumCodeine • Morphine • Oripavine • Pseudomorphine • Thebaine
Alkaloid salts mixtures Semisynthetics
including
Bentley compoundsMorphine family 3,6-diesters of morphine Acetylpropionylmorphine • Diacetyldihydromorphine (Dihydroheroin, Acetylmorphinol) • Dibenzoylmorphine • Dipropanoylmorphine • Heroin (Diacetylmorphine) • Nicomorphine
Codeine-dionine family Morphinones and morphols 14-Cinnamoyloxycodeinone • 14-Ethoxymetopon • 14-Methoxymetopon • 14-Phenylpropoxymetopon • 7-Spiroindanyloxymorphone • Acetylmorphone • Codeinone • Codorphone • Codoxime • Thebacon (Acetyldihydrocodeinone / Dihydrocodeinone enol acetate) • Hydrocodone • Hydromorphone • Metopon • Morphinone • N-Phenethyl-14-ethoxymetopon • Oxycodone • Oxymorphol • Oxymorphone • Pentamorphone • Semorphone
Morphides Dihydrocodeine series 14-hydroxydihydrocodeine • Acetyldihydrocodeine • Dihydrocodeine • Nicocodeine • Nicodicodeine
Nitrogen morphine derivatives Hydrazones Halogenated morphine derivatives 1-Iodomorphine
Active opiate
metabolitesMorphine-6-glucuronide • 6-Monoacetylmorphine • Norcodeine • Normorphine
Morphinans Morphinan series Cyclorphan • Dextrallorphan • Levorphanol • Levophenacylmorphan • Levomethorphan • Norlevorphanol • Oxilorphan • Phenomorphan • Methorphan / Racemethorphan • Morphanol / Racemorphanol • Ro4-1539 • Xorphanol
Others Benzomorphans 8-Carboxamidocyclazocine • Alazocine • Bremazocine • Dezocine • Ketazocine • Metazocine • Pentazocine • Phenazocine
4-Phenylpiperidines Pethidines
(Meperidines)4-Fluoromeperidine • Allylnorpethidine • Anileridine • Benzethidine • Carperidine • Difenoxin • Diphenoxylate • Etoxeridine (Carbetidine) • Furethidine • Hydroxypethidine (Bemidone) • Morpheridine • Oxpheneridine (Carbamethidine) • Pethidine (Meperidine) • Pethidine Intermediate A • Pethidine Intermediate B (Norpethidine) • Pethidine Intermediate C (Pethidinic Acid) • Pheneridine • Phenoperidine • Piminodine • Properidine (Ipropethidine) • Sameridine
Prodines Ketobemidones Others Open chain
opioidsAmidones Methadols Moramides Dextromoramide • Levomoramide • Racemoramide
Thiambutenes Phenalkoxams Ampromides Others IC-26 • Isoaminile • Lefetamine • R-4066
Anilidopiperidines 3-Allylfentanyl • 3-Methylfentanyl • 3-Methylthiofentanyl • 4-Phenylfentanyl • Alfentanil • α-methylacetylfentanyl • α-methylfentanyl • α-methylthiofentanyl • β-hydroxyfentanyl • β-hydroxythiofentanyl • β-methylfentanyl • Brifentanil • Carfentanil • Fentanyl • Lofentanil • Mirfentanil • Ocfentanil • Ohmefentanyl • Parafluorofentanyl • Phenaridine • Remifentanil • Sufentanil • Thiofentanyl • Trefentanil
Oripavine
derivativesPhenazepanes Pirinitramides Benzimidazoles Indoles Diphenylmethylpiperazines Opioid peptides
see also: Opioid neuropeptidesAdrenorphin • Amidorphin • Casomorphin • DADLE • DAMGO • Dermorphin • Dynorphin • Endomorphin • Endorphins • Enkephalin • Gliadorphin • Morphiceptin • Nociceptin • Octreotide • Opiorphin • Rubiscolin • TRIMU 5
Others AD-1211 • AH-7921 • Azaprocin • BDPC • BRL-52537 • Bromadoline • C-8813 • Ciramadol • Doxpicomine • Enadoline • Faxeladol • GR-89696 • Herkinorin • ICI-199,441 • ICI-204,448 • J-113,397 • JTC-801 • Ketamine • LPK-26 • Methopholine • MT-45 • N-Desmethylclozapine • NNC 63-0532 • Nortilidine • O-Desmethyltramadol • Phencyclidine • Prodilidine • Profadol • Ro64-6198 • Salvinorin A • SB-612,111 • SC-17599 • RWJ-394,674 • TAN-67 • Tapentadol • Tifluadom • Tilidine • Tramadol • Trimebutine • U-50,488 • U-69,593 • Viminol • W-18
Opioid antagonists
&
inverse agonists5'-Guanidinonaltrindole • Alvimopan • Chlornaltrexamine • Cyclazocine • Cyprodime • Diprenorphine (M5050) • JDTic • Levallorphan • Methylnaltrexone • Nalfurafine • Nalmefene • Naloxazone • Naloxonazine • Naloxone • Nalorphine • Naltrexol-d4 • Naltrexone • Naltriben • Naltrindole • Norbinaltorphimine • Oxilorphan
List of opioidsCategories:- Antidiarrhoeals
- Piperidines
- Alcohols
- Organochlorides
- Synthetic opioids
- Butyramides
- Mu-opioid agonists
- Janssen Pharmaceutica
Wikimedia Foundation. 2010.