- Nifuroxazide
-
Nifuroxazide 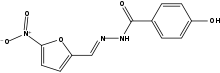
Systematic (IUPAC) name 4-Hydroxy-N'-[(5-nitrofuran-2-yl)methylene]benzohydrazide Clinical data AHFS/Drugs.com International Drug Names Pregnancy cat. ? Legal status ? Routes Oral Identifiers CAS number 965-52-6 
ATC code A07AX03 PubChem CID 5337997 ChemSpider 4495115 
UNII PM5LI0P38J 
KEGG D07111 
ChEMBL CHEMBL244888 
Chemical data Formula C12H9N3O5 Mol. mass 275.2 g/mol SMILES eMolecules & PubChem  (what is this?) (verify)
(what is this?) (verify)Nifuroxazide (INN) is an oral nitrofuran antibiotic, patented since 1966[1] and used to treat colitis and diarrhea in humans and non-humans.[2] It is sold under the brand names Ambatrol, Antinal, Bacifurane, Diafuryl (FR), Nifrozid, Ercefuryl, Erfuzide (TH), Endiex,Endiaron, Ercefurin,Nifuroksazyd (CZ, SK, PL), Pérabacticel (FR), Pentofuryl (DE) Enteral (Afrique Norte) and Septidiaryl. It is sold in capsule form and also as a suspension. The pharmaceutical group SmithKline Beecham claims that nifuroxazide is highly effective and the consumers' group Healthy Skepticism says that SmithKline Beecham's claims have no scientific support.[3]
Contents
History
Maurice Claude Ernest Carron patented the drug in the United States in 1966 [1]. Subsequent patents issued to Germano Cagliero of Marxer S.p.A describe the use of nifuroxazide as an antibiotic used to treat livestock.[2]
Effectiveness in humans
In 1997, in an Ivory Coast promotional leaflet, SmithKline Beecham claimed that nifuroxazide (under the brand name "Ambatrol") is an anti-dehydration treatment, "neutralise[s] microbacterials" in diarrhoea, and has "a spectrum which covers most enteropathogenic microbacterials, Shigella, Escherichia Coli, Salmonella, Staphylococci, Klebsiella, Yersinia".[3] The international non-profit organisation Healthy Skepticism, at the time using their former name, Medical Lobby for Appropriate Marketing (MaLAM), disagreed, stating "We have not found any scientific evidence to support these claims."[3]
Notes
- ^ a b USPTO No. 3290213 |http://www.google.com/patents?id=f2dwAAAAEBAJ
- ^ a b USPTO No 4093746 |http://www.google.com/patents?vid=USPAT4093746
- ^ a b c "SmithKline Beecham Ambatrol (nifuroxazide)". Healthy Skepticism. 1997-06. Archived from the original on 2010-12-21. http://www.healthyskepticism.org/global/malam/int/mi1997-05_06/. Retrieved 2010-12-21.
External links

This systemic antibacterial-related article is a stub. You can help Wikipedia by expanding it. This drug article relating to the gastrointestinal system is a stub. You can help Wikipedia by expanding it.
