- Mesalazine
-
Mesalazine 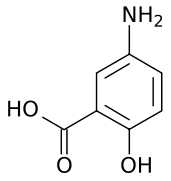
Systematic (IUPAC) name 5-amino-2-hydroxybenzoic acid Clinical data AHFS/Drugs.com monograph Pregnancy cat. B(US) Legal status ℞-only (US) Routes oral, rectal Pharmacokinetic data Bioavailability orally: 20-30% absorbed
rectally: 10-35%Metabolism Rapidly & extensively metabolised intestinal mucosal wall and the liver Half-life 5 hours after initial dose.
At steady state 7 hoursIdentifiers CAS number 89-57-6 
ATC code A07EC02 PubChem CID 4075 DrugBank APRD01098 ChemSpider 3933 
UNII 4Q81I59GXC 
KEGG D00377 
ChEBI CHEBI:6775 
ChEMBL CHEMBL704 
Chemical data Formula C7H7NO3 Mol. mass 153.135 g/mol SMILES eMolecules & PubChem  (what is this?) (verify)
(what is this?) (verify)Mesalazine (INN, BAN), also known as Mesalamine (USAN) or 5-aminosalicylic acid (5-ASA), is an anti-inflammatory drug used to treat inflammation of the digestive tract ulcerative colitis[1] and mild-to-moderate Crohn's disease.[2] Mesalazine is a bowel-specific aminosalicylate drug that acts locally in the gut and has its predominant actions there, thereby having few systemic side effects.[3] As a derivative of salicylic acid, 5-ASA is also thought to be an antioxidant that traps free radicals, which are potentially damaging byproducts of metabolism.[4]
5-ASA is considered the active moiety of sulfasalazine, which is metabolized to sulfapyridine and 5_ASA.[5]
Contents
Formulations
Mesalazine is formulated for oral ingestion as tablets or granules, and for rectal administration as a rectal suppository, suspension or enemas. It is marketed under a variety of brand names:
- UK: Asacol, Ipocal, Pentasa, Salofalk, Mezavant XL
- France: Asacol, Pentasa, Mezavant
- US: Canasa, Rowasa, Pentasa, Asacol, Lialda, Apriso
- Canada: Asacol, Pentasa, Salofalk, Mezavant
- India: Mesacol
- Mexico: Salofalk
The newest of these is Apriso (Salofalk granules in Europe), approved by the U.S. Food and Drug Administration (FDA) in October 2008 for the induction and maintenance of remission in ulcerative colitis. Its main benefit is that it needs to be taken only once a day, which provides convenient dosing regimen for patients. Several formulations of mesalazine have published data to suggest that once-daily dosing is sufficient in ulcerative colitis.
Lialda contains the highest mesalamine dose per tablet (1.2 g).
Dosing depends on the preparation used; in particular, slow-release tablets may have quite different drug delivery characteristics and are not interchangeable.
Preparations that lower stool pH (such as lactulose, a laxative) will possibly affect the binding of mesalazine in the bowel and will therefore reduce its efficacy.
Side effects
Commonly:
- Diarrhea
- Nausea
- Cramping
- Flatulence[6]
Uncommonly:
- Headache
- Exacerbation of the colitis
- Hypersensitivity reactions (including rash, urticaria aka hives, interstitial nephritis and lupus erythematosus-like syndrome)
- Hair Loss
- Interstitial nephritis
Rarely:
- Acute pancreatitis
- Hepatitis
- Nephrotic syndrome
- Blood disorders (including agranulocytosis, aplastic anaemia, leukopenia, neutropenia, thrombocytopenia)
Mesalazine avoids the sulphonamide side effects of sulfasalazine (which contains additional sulfapyridine), but carries additional rare risks of:
- Allergic lung reactions
- Allergic myocarditis
- Methaemoglobinaemia
Monitoring
As a result of the small risks of kidney, liver and blood disorders, blood tests should be taken before and after starting treatment. Patients are advised to report any unexplained bleeding, bruising, purpura, sore throat, fever or malaise that occurs during treatment so that a full blood count can be urgently taken.
References
- ^ Kruis, W; Schreiber, I; Theuer; Brandes; Schütz; Howaldt; Krakamp; Hämling et al. (2001). "Low dose balsalazide (1.5 g twice daily) and mesalazine (0.5 g three times daily) maintained remission of ulcerative colitis but high dose balsalazide (3.0 g twice daily) was superior in preventing relapses". Gut 49 (6): 783–9. doi:10.1136/gut.49.6.783. PMC 1728533. PMID 11709512. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=1728533.
- ^ Sandborn WJ, Feagan BG, Lichtenstein GR (October 2007). "Medical management of mild to moderate Crohn's disease: evidence-based treatment algorithms for induction and maintenance of remission". Alimentary Pharmacology & Therapeutics 26 (7): 987–1003. doi:10.1111/j.1365-2036.2007.03455.x. PMID 17877506. http://www3.interscience.wiley.com/cgi-bin/fulltext/117987903/HTMLSTART. Retrieved 2009-12-20.
- ^ http://www.pharmgkb.org/do/serve?objCls=Drug&objId=PA450384#tabview=tab1
- ^ http://www.pharmgkb.org/do/serve?objCls=Drug&objId=PA450384#tabview=tab1
- ^ Lippencott's Illustrated Reviews: Pharmacology, 4th Ed. Finkel, Cubeddu and Clark.
- ^ "Lialda Side Effects & Safety Information". Shire US. October 2007. http://www.lialda.com/aboutLialda/sideEffect.asp. Retrieved 2008-01-07.
- Mehta, Dinesh K, ed (March 2003). British National Formulary. 45. London: Pharmaceutical Press. ISBN 0853695555.
- Sweetman, Sean C, ed (November 30, 2004). Martindale: The complete drug reference (34th ed.). London: Pharmaceutical Press. ISBN 0-85369-550-4.
External links
- Optimal Dosing of 5-Aminosalicylic Acid: 5 Decades of Choosing Between Politicians
- "Novel formulation increases efficacy of mesalamine for treating ulcerative colitis" Reuters article on Lialda/Mezavant trial success.
- Once daily mesalazine effective in active ulcerative colitis: study
- Pentasa Official Site
- Asacol Official Site
- Lialda Official Site
- Apriso Official Site
Categories:- Anti-inflammatory agents
- Gastroenterology
- Salicylic acids
- Anilines
Wikimedia Foundation. 2010.