- Neomycin
-
Neomycin 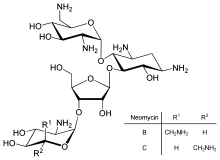
Systematic (IUPAC) name (1R,2R,3S,4R,6S)-4,6-diamino-2- Clinical data Trade names Neo-rx AHFS/Drugs.com monograph MedlinePlus a682274 Pregnancy cat. ? Legal status OTC Routes Topical, Oral Pharmacokinetic data Half-life 2 to 3 hours Identifiers CAS number 1404-04-2 ATC code A01AB08 A07AA01, B05CA09, D06AX04, J01GB05, R02AB01, S01AA03, S02AA07, S03AA01 PubChem CID 8378 IUPHAR ligand 709 DrugBank DB00994 ChemSpider 8075 UNII I16QD7X297 
KEGG D08260 ChEBI CHEBI:7508 ChEMBL CHEMBL449118 Chemical data Formula C23H46N6O13 Mol. mass 614.644 g/mol SMILES eMolecules & PubChem - InChI=1S/C23H46N6O13/c24-2-7-13(32)15(34)10(28)21(37-7)40-18-6(27)1-5(26)12(31)20(18)42-23-17(36)19(9(4-30)39-23)41-22-11(29)16(35)14(33)8(3-25)38-22/h5-23,30-36H,1-4,24-29H2/t5-,6+,7-,8+,9-,10-,11-,12+,13-,14-,15-,16-,17-,18-,19-,20-,21-,22-,23+/m1/s1
Key:PGBHMTALBVVCIT-VCIWKGPPSA-N
Neomycin is an aminoglycoside antibiotic that is found in many topical medications such as creams, ointments, and eyedrops. The discovery of Neomycin dates back to 1949. It was discovered in the lab of Selman Waksman, who was later awarded the Nobel Prize in Physiology and medicine in 1951. Neomycin, belongs to aminoglycoside class of antibiotics which contain two or more aminosugars connected by glycosidic bonds. Neamine (two rings), Ribostamycin (three rings), Paromomycin (four rings) and Lividomycin (five rings) are some other examples of aminoglycosides. They have shown tremendous potential as antibacterials. One of them, Gentamicin has been used extensively in clinical practice. Due to the inherent oto and nephrotoxicity of these substances, systemic use has declined as safer alternatives have become available.
Contents
Uses
Neomycin is overwhelmingly used as a topical preparation, such as Neosporin. It can also be given orally, where it is usually combined with other antibiotics. Neomycin is not absorbed from the gastrointestinal tract and has been used as a preventive measure for hepatic encephalopathy and hypercholesterolemia. By killing bacteria in the intestinal tract, it keeps ammonia levels low and prevents hepatic encephalopathy, especially prior to GI surgery. It has also been used to treat small intestinal bacterial overgrowth. It is not given intravenously, as neomycin is extremely nephrotoxic (causes kidney damage), especially compared to other aminoglycosides. The exception is when neomycin is included, in very small quantities, as a preservative in some vaccines - typically 0.025 mg per dose.[1]
Molecular biology
Neomycin resistance is conferred by either one of two aminoglycoside phosphotransferase genes.[2] A neo gene is commonly included in DNA plasmids used by molecular biologists to establish stable mammalian cell lines expressing cloned proteins in culture; many commercially available protein expression plasmids contain neo as a selectable marker. Non-transfected cells will eventually die off when the culture is treated with neomycin or similar antibiotic. Neomycin or kanamycin can be used for prokaryotes, but geneticin (G418) is, in general, needed for eukaryotes.
Spectrum
Similar to other aminoglycosides, neomycin has excellent activity against Gram-negative bacteria, and has partial activity against Gram-positive bacteria. It is relatively toxic to humans, and many people have allergic reactions to it.[3] See: Hypersensitivity. Physicians sometimes recommend using antibiotic ointments without neomycin, such as Polysporin.[4]
History
Neomycin was discovered in 1949 by the microbiologist Selman Waksman and his student Hubert Lechevalier at Rutgers University. It is produced naturally by the bacterium Streptomyces fradiae.[5]
Neomycin as a DNA binder
Neomycin belongs to the family of aminoglycosides. This family includes many other medicinally important drugs: streptomycin, paromomycin and kanamycin . Aminoglycosides are known for their ability to bind to duplex RNA with high affinity. A study done by Daniel Pilch, Associate Professor Dept. of Pharmacology at Rutgers University, and his coworkers determined the association constant for neomycin with A-site RNA was found to be in the ~109 range. However, more than 50 years after its discovery, its DNA-binding properties were still unknown. In 2000, Dev P. Arya, currently Director of the Laboratory of Medicinal Chemistry at Clemson University, and his coworkers discovered that neomycin induces enormous thermal stabilization of triplex DNA while having little or almost no effect on the DNA duplex stabilization. They also showed that neomycin binds to structures that adopt A-form structure, triplex DNA being one of them. They later went on to show that neomycin even induces DNA:RNA hybrid triplex formation. [6]
References
- ^ "Medscape article". http://www.medscape.com/viewarticle/516045_6.
- ^ "G418/neomycin-cross resistance?". http://www.bio.net/bionet/mm/methods/1999-March/073912.html. Retrieved 2008-10-19.
- ^ DermNet dermatitis/neomycin-allergy
- ^ "Your Medicine Cabinet". DERMAdoctor.com, Inc.. http://www.dermadoctor.com/article_Your-Medicine-Cabinet_43.html. Retrieved 2008-10-19.
- ^ "The Nobel Prize in Physiology or Medicine 1952". Nobel Foundation. http://nobelprize.org/nobel_prizes/medicine/laureates/1952/waksman-bio.html. Retrieved 2008-10-29.
- ^ http://www.wiley-vch.de/publish/en/books/bySubjectCH00/ISBN0-471-74302-X/?sID=5vh8aprb95023mtg84ga23gih0
Stomatological preparations (A01) Caries prophylactic agents Anti-infectives and antiseptics Amphotericin B • Benzoxonium chloride • Chlorhexidine • Chlortetracycline • Clotrimazole • Domiphen bromide • Doxycycline • Eugenol • Hexetidine • Hydrogen peroxide • Mepartricin • Metronidazole • Miconazole • Minocycline • Natamycin • Neomycin • Oxyquinoline • Polynoxylin • Sodium perborate • Tetracycline • Tibezonium iodideCorticosteroids (Glucocorticoids) Other Antibiotics and chemotherapeutics for dermatological use (D06) Antibiotics Tetracycline and derivativesOthersAminoglycosides: Neomycin • Gentamicin • Amikacin
other: Fusidic acid • Bacitracin • Tyrothricin • MupirocinChemotherapeutics Aciclovir • Penciclovir • Idoxuridine • Edoxudine
Imiquimod/Resiquimod • Podophyllotoxin
Docosanol • Tromantadine • Inosine • Lysozyme • IbacitabineOtherAntibacterials: protein synthesis inhibitors (J01A, J01B, J01F, J01G, QJ01XQ) 30S -mycin (Streptomyces)Neomycin# (Framycetin, Paromomycin, Ribostamycin)
Kanamycin# (Amikacin, Arbekacin, Bekanamycin, Dibekacin, Tobramycin)
Paromomycin-micin (Micromonospora)Tetracyclines50S Linezolid • Torezolid • Eperezolid • Posizolid • RadezolidPleuromutilinsRetapamulin • Tiamulin • ValnemulinErythromycin# • Azithromycin# • Spiramycin • Midecamycin • Oleandomycin • Roxithromycin • Josamycin • Troleandomycin • Clarithromycin • Miocamycin • Rokitamycin • Dirithromycin • Flurithromycin • Ketolide (Telithromycin, Cethromycin, Solithromycin)EF-G Steroid antibacterialsThroat preparations (R02) Antiseptics Acriflavinium chloride • Ambazone • Benzalkonium • Benzethonium • Cetrimonium (bromide/chloride) • Cetylpyridinium • Chlorhexidine • Chlorquinaldol • Dequalinium • Dichlorobenzyl alcohol • Hexamidine • Hexylresorcinol • Myristyl-benzalkonium • Oxyquinoline • Phenol • Povidone-iodineAntibiotics Local anesthetics Other Otologicals (S02) Anti-infectives Acetic acid • Aluminium acetotartrate • Boric acid • Chloramphenicol • Chlorhexidine • Ciprofloxacin • Clioquinol • Gentamicin • Hydrogen peroxide • Miconazole • Neomycin • Nitrofurazone • Ofloxacin • Polymyxin B • Rifamycin • TetracyclineCorticosteroids Analgesics and anesthetics M: EAR
anat(e/p)/phys/devp
noco/cong, epon
proc, drug(S2)
Categories:- Aminoglycoside antibiotics
- Otologicals
- InChI=1S/C23H46N6O13/c24-2-7-13(32)15(34)10(28)21(37-7)40-18-6(27)1-5(26)12(31)20(18)42-23-17(36)19(9(4-30)39-23)41-22-11(29)16(35)14(33)8(3-25)38-22/h5-23,30-36H,1-4,24-29H2/t5-,6+,7-,8+,9-,10-,11-,12+,13-,14-,15-,16-,17-,18-,19-,20-,21-,22-,23+/m1/s1
Wikimedia Foundation. 2010.
