- Idoxuridine
-
Idoxuridine 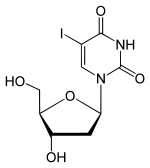
Systematic (IUPAC) name 1-[(2R,4S,5R)-4-hydroxy-5-(hydroxymethyl)oxolan-2-yl]-5-iodo-1,2,3,4-tetrahydropyrimidine-2,4-dione Clinical data AHFS/Drugs.com Micromedex Detailed Consumer Information MedlinePlus a601062 Pregnancy cat. ? Legal status ? Routes intravenously Identifiers CAS number 54-42-2 
ATC code D06BB01 J05AB02, S01AD01 PubChem CID 5905 DrugBank APRD00504 ChemSpider 10481938 
UNII LGP81V5245 
KEGG D00342 
ChEMBL CHEMBL788 
Synonyms Iododeoxyuridine; IUdR Chemical data Formula C9H11IN2O5 Mol. mass 354.099 g/mol SMILES eMolecules & PubChem  (what is this?) (verify)
(what is this?) (verify)Idoxuridine is an anti-herpesvirus antiviral drug.
It is a nucleoside analogue, a modified form of deoxyuridine, similar enough to be incorporated into viral DNA replication, but the iodine atom added to the uracil component blocks base pairing. It is used only topically due to cardiotoxicity.
Clinical use
Idoxuridine is mainly used topically to treat herpes simplex keratitis.[1] Epithelial lesions, especially initial attacks presenting with a dendritic ulcer, are most responsive to therapy, while infection with stromal involvement are less responsive.[2] Idoxuridine is ineffective against herpes simplex virus type 2 and varicella-zoster.[1]
Formulations and dosage
Idoxuridine is available as either a 0.5% ophthalmic ointment or as a 0.1% ophthalmic solution.[1] The dosage of the ointment is every 4 hours during day and once before bedtime.[1] The dosage of the solution is 1 drop in the conjunctival sac hourly during the day and every 2 hours during the night until definitive improvement, then 1 drop every 2 hours during the day and every 4 hours during the night.[1] Therapy is continued for 3-4 days after healing is complete, as demonstrated by fluorescein staining.[1]
References
- Seth A, Misra A, Umrigar D (2004). "Topical liposomal gel of idoxuridine for the treatment of herpes simplex: pharmaceutical and clinical implications". Pharm Dev Technol 9 (3): 277–289. doi:10.1081/PDT-200031432. PMID 15458233.
- Otto S (1998). "Radiopharmaceuticals (Strontium 89) and radiosensitizers (idoxuridine)". J Intraven Nurs 21 (6): 335–7. PMID 10392098.
- Fauth E, Zankl H (1999). "Comparison of spontaneous and idoxuridine-induced micronuclei by chromosome painting". Mutat Res 440 (2): 147–56. PMID 10209337.
- ^ a b c d e f Goodman and Gilman's The Pharmacological Basis of Therapeutics. Edited by Gilman AG, Rall TW, Nies AS, Taylor P. McGraw-Hill. 8th ed. 1990.
- ^ Maxwell E. Treatment of herpes keratitis with 5-iodo-2-deoxyuridine (IDU): a clinical evaluation of 1500 cases. Am. J. Ophthalmol., 1963, 56, 571-573.
Antibiotics and chemotherapeutics for dermatological use (D06) Antibiotics Tetracycline and derivativesOthersChemotherapeutics Aciclovir • Penciclovir • Idoxuridine • Edoxudine
Imiquimod/Resiquimod • Podophyllotoxin
Docosanol • Tromantadine • Inosine • Lysozyme • IbacitabineOtherDNA virus antivirals (primarily J05, also S01AD and D06BB) Baltimore I DNA-synthesis
inhibitorTK activatedguanine (Aciclovir#/Valacyclovir, Ganciclovir/Valganciclovir, Penciclovir/Famciclovir)
adenine (Vidarabine)Not TK activatedOtherImiquimod/Resiquimod • PodophyllotoxinHepatitis B (VII) Nucleoside analogues/NARTIs: Entecavir • Lamivudine • Telbivudine • Clevudine
Nucleotide analogues/NtRTIs: Adefovir • TenofovirMultiple/general Nucleic acid inhibitorsMultiple/unknownErbB2/PI3K PathwayNOV-205§ • NOV-002†
This antiinfective drug article is a stub. You can help Wikipedia by expanding it. This dermatologic drug article is a stub. You can help Wikipedia by expanding it.
