- Clevudine
-
Clevudine 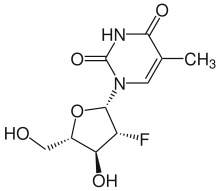
Systematic (IUPAC) name 1-[(2S,3R,4S,5S)-3-fluoro-4-hydroxy-5- (hydroxymethyl)oxolan-2-yl]-5-methylpyrimidine- 2,4-dione Clinical data Pregnancy cat. ? Legal status ? Routes Oral Identifiers CAS number 69256-17-3 
ATC code J05AF12 [1] PubChem CID 73115 UNII IN51MVP5F1 
KEGG D03537 
ChEMBL CHEMBL458875 
Chemical data Formula C10H13FN2O5 Mol. mass 260.219 g/mol  (what is this?) (verify)
(what is this?) (verify)Clevudine (INN) is an antiviral drug for the treatment of hepatitis B. Clevudine is already approved for HBV in South Korea and the Philippines. It is marketed by Bukwang pharmaceuticals in South Korea under the tradenames Levovir and Revovir. Under license from Bukwang, Pharmasset was developing the drug, but its phase III clinical trial (international, multicenter, randomized, double-blind, 96 week QUASH studies) was terminated due to some myopathy cases in patients.
It is a nucleoside analog.[2]
References
- ^ WHO International Working Group for Drug Statistics Methodology (August 27, 2008). "ATC/DDD Classification (FINAL): New ATC 5th level codes". WHO Collaborating Centre for Drug Statistics Methodology. Archived from the original on 2008-05-06. http://web.archive.org/web/20080506023243/http://www.whocc.no/atcddd/new_atc_ddd.html#ATCDDD_FINAL. Retrieved 2008-09-05.
- ^ Lee HS, Chung YH, Lee K, et al. (May 2006). "A 12-week clevudine therapy showed potent and durable antiviral activity in HBeAg-positive chronic hepatitis B". Hepatology 43 (5): 982–8. doi:10.1002/hep.21166. PMID 16628625.
DNA virus antivirals (primarily J05, also S01AD and D06BB) Baltimore I DNA-synthesis
inhibitorTK activatedguanine (Aciclovir#/Valacyclovir, Ganciclovir/Valganciclovir, Penciclovir/Famciclovir)
adenine (Vidarabine)Not TK activatedOtherImiquimod/Resiquimod • PodophyllotoxinHepatitis B (VII) Nucleoside analogues/NARTIs: Entecavir • Lamivudine • Telbivudine • Clevudine
Nucleotide analogues/NtRTIs: Adefovir • TenofovirMultiple/general Nucleic acid inhibitorsMultiple/unknownErbB2/PI3K PathwayNOV-205§ • NOV-002†
This antiinfective drug article is a stub. You can help Wikipedia by expanding it.
