- Cidofovir
-
Cidofovir 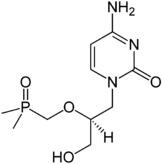
Systematic (IUPAC) name ({[(S)-1-(4-amino-2-oxo-1,2-dihydropyrimidin-1-yl)-3-hydroxypropan-2-yl]oxy}methyl)phosphonic acid Clinical data Trade names Vistide AHFS/Drugs.com monograph Pregnancy cat. ? Legal status ? Routes intravenous Pharmacokinetic data Bioavailability complete Protein binding 6% Half-life 2.4 to 3.2 hours Excretion renal Identifiers CAS number 113852-37-2 ATC code J05AB12 PubChem CID 60613 DrugBank APRD00148 ChemSpider 54636 
NIAID ChemDB 001049 UNII 768M1V522C 
ChEBI CHEBI:3696 
ChEMBL CHEMBL152 
Chemical data Formula C8H14N3O6P Mol. mass 279.187 g/mol SMILES eMolecules & PubChem Physical data Melt. point 260 °C (500 °F) Spec. rot -97.3  (what is this?) (verify)
(what is this?) (verify)Cidofovir is an injectable antiviral medication for the treatment of cytomegalovirus (CMV) retinitis[1] in patients with AIDS. It suppresses CMV replication by selective inhibition of viral DNA polymerase and therefore prevention of viral replication and transcription.[2] It is an acyclic nucleoside phosphonate, and is therefore independent of phosphorylation by viral enzymes,[3] in contrast to, for instance, acyclovir.
Contents
Administration
Maintenance therapy with cidofovir involves an infusion only once every two weeks, making it a convenient treatment option. Because dosing is relatively infrequent, a permanent catheter is not necessary for infusions.
Side effects
The major side effect of cidofovir is that it can be nephrotoxic.[4]
Probenecid (a uricosuric drug) is usually prescribed to prevent this nephrotoxicity.
Uses
DNA virus
Cidofovir demonstrated a statistically significant effect in delaying the progression of CMV retinitis lesions in newly diagnosed patients, as well as in previously treated patients who had failed other therapies.
Cidofovir has shown efficacy as an anti-HSV, anti-VZV and as an anti-CMV
Cidofovir has also shown efficacy in the treatment of acyclovir resistant herpes
Cidofovir has also been investigated as a treatment for progressive multifocal leukoencephalopathy,[5] but as of 2005[update] studies are inconclusive.
Cidofovir might have anti-smallpox efficacy[6] and might be used on a limited basis in the event of a bioterror incident involving smallpox cases.In fact, it is a extremely high chance for Cidofovir to work against smallpox.
Cidofovir shows anti-BK virus activity in a subgroup of transplant patients.[7]
Cidofovir is being investigated as a complementary intralesional therapy against papillomatosis caused by HPV.[8][9]
Other
It has been suggested as an antitumor agent, due to its suppression of FGF2.[10][11]
History
Cidofovir was discovered at the Institute of Organic Chemistry and Biochemistry, Prague, by Antonín Holý, and developed by Gilead Sciences[12] and is marketed with the brand name Vistide by Gilead in the USA, and by Pfizer elsewhere.
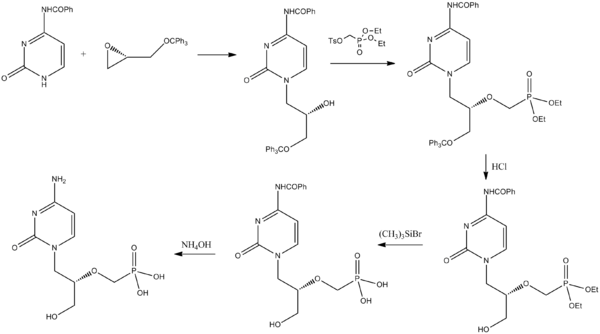
Synthesis
Brodfuehrer, P (1994). "A practical synthesis of (S)-HPMPC". Tetrahedron Letters 35: 3243. doi:10.1016/S0040-4039(00)76875-4.
References
- ^ Becker MN, Obraztsova M, Kern ER, et al. (2008). "Isolation and characterization of cidofovir resistant vaccinia viruses". Virol. J. 5: 58. doi:10.1186/1743-422X-5-58. PMC 2397383. PMID 18479513. http://www.virologyj.com/content/5//58.
- ^ Cidofovir VIRUSES, HIV, PRIONS, AND RELATED TOPICS. Human Virology at Stanford University
- ^ The mechanism of action of cidofovir and HSV helicase–primase complex inhibitors. Nature reviews
- ^ Kazory A, Singapuri S, Wadhwa A, Ejaz AA (July 2007). "Simultaneous development of Fanconi syndrome and acute renal failure associated with cidofovir". J. Antimicrob. Chemother. 60 (1): 193–4. doi:10.1093/jac/dkm143. PMID 17496056. http://jac.oxfordjournals.org/cgi/pmidlookup?view=long&pmid=17496056.
- ^ Segarra-Newnham M, Vodolo KM (June 2001). "Use of cidofovir in progressive multifocal leukoencephalopathy". Ann Pharmacother 35 (6): 741–4. doi:10.1345/aph.10338. PMID 11408993. http://www.theannals.com/cgi/pmidlookup?view=long&pmid=11408993.
- ^ De Clercq E (July 2002). "Cidofovir in the treatment of poxvirus infections". Antiviral Res. 55 (1): 1–13. doi:10.1016/S0166-3542(02)00008-6. PMID 12076747. http://linkinghub.elsevier.com/retrieve/pii/S0166354202000086.
- ^ Araya CE, Lew JF, Fennell RS, Neiberger RE, Dharnidharka VR (February 2006). "Intermediate-dose cidofovir without probenecid in the treatment of BK virus allograft nephropathy". Pediatr Transplant 10 (1): 32–7. doi:10.1111/j.1399-3046.2005.00391.x. PMID 16499584. http://www3.interscience.wiley.com/resolve/openurl?genre=article&sid=nlm:pubmed&issn=1397-3142&date=2006&volume=10&issue=1&spage=32.
- ^ Broekema FI, Dikkers FG (August 2008). "Side-effects of cidofovir in the treatment of recurrent respiratory papillomatosis". Eur Arch Otorhinolaryngol 265 (8): 871–9. doi:10.1007/s00405-008-0658-0. PMC 2441494. PMID 18458927. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2441494.
- ^ Soma MA, Albert DM (February 2008). "Cidofovir: to use or not to use?". Curr Opin Otolaryngol Head Neck Surg 16 (1): 86–90. doi:10.1097/MOO.0b013e3282f43408. PMID 18197029. http://meta.wkhealth.com/pt/pt-core/template-journal/lwwgateway/media/landingpage.htm?an=00020840-200802000-00019.
- ^ Liekens S, Gijsbers S, Vanstreels E, Daelemans D, De Clercq E, Hatse S (March 2007). "The nucleotide analog cidofovir suppresses basic fibroblast growth factor (FGF2) expression and signaling and induces apoptosis in FGF2-overexpressing endothelial cells". Mol. Pharmacol. 71 (3): 695–703. doi:10.1124/mol.106.026559. PMID 17158200. http://molpharm.aspetjournals.org/cgi/pmidlookup?view=long&pmid=17158200.
- ^ Liekens S (2008). "Regulation of cancer progression by inhibition of angiogenesis and induction of apoptosis". Verh. K. Acad. Geneeskd. Belg. 70 (3): 175–91. PMID 18669159.
- ^ "Press Releases: Gilead". http://www.gilead.com/pr_881577. Retrieved 2007-12-05.
DNA virus antivirals (primarily J05, also S01AD and D06BB) Baltimore I DNA-synthesis
inhibitorTK activatedguanine (Aciclovir#/Valacyclovir, Ganciclovir/Valganciclovir, Penciclovir/Famciclovir)
adenine (Vidarabine)Not TK activatedOtherHepatitis B (VII) Nucleoside analogues/NARTIs: Entecavir • Lamivudine • Telbivudine • Clevudine
Nucleotide analogues/NtRTIs: Adefovir • TenofovirMultiple/general Nucleic acid inhibitorsCidofovirMultiple/unknownErbB2/PI3K PathwayNOV-205§ • NOV-002†Categories:- Gilead Sciences
- Antivirals
- Pyrimidones
- Amines
- Ethers
- Phosphonic acids
- Alcohols
Wikimedia Foundation. 2010.