- Podophyllotoxin
-
Podophyllotoxin 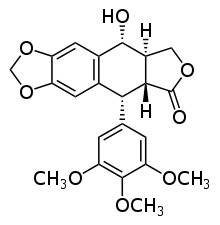
Systematic (IUPAC) name (10R,11R,15R,16R)-16-hydroxy-10-(3,4,5-trimethoxyphenyl)-4,6,13-trioxatetracyclo[7.7.0.03,7.011,15]hexadeca-1,3(7),8-trien-12-one Clinical data AHFS/Drugs.com International Drug Names MedlinePlus a684055 Pregnancy cat. ? Legal status ? Pharmacokinetic data Half-life 1.0 to 4.5 hours. Identifiers CAS number 518-28-5 
ATC code D06BB04 PubChem CID 10607 DrugBank APRD01189 ChemSpider 10162 
UNII L36H50F353 
ChEBI CHEBI:50305 
ChEMBL CHEMBL61 
Synonyms (5R,5aR,8aR,9R)-9-hydroxy-5-(3,4,5-trimethoxyphenyl)-5,8,8a,9-tetrahydrofuro[3',4':6,7]naphtho[2,3-d][1,3]dioxol-6(5aH)-one Chemical data Formula C22H22O8 Mol. mass 414.405 g/mol SMILES eMolecules & PubChem  (what is this?) (verify)
(what is this?) (verify)Podophyllotoxin (abbreviated as PPT), otherwise known as podofilox, is a non-alkaloid toxin lignan extracted from the roots and rhizomes of Podophyllum species[1]. Under the trade name Condylox, a topical gel, it is used on the skin to treat external genital warts, caused by some types of the human papillomavirus (HPV). PPT and its derivatives display a wide selection in medical applications such as purgative, vesicant, antirheumatic, antiviral, and antitumor agents. These derivatives include etoposide, teniposide, and etopophos. Their anticancer activity has been heavily under study and used in various chemotherapies, including lung cancer, lymphomas, and genital tumors.
Contents
Natural abundance
It is present at concentrations of 0.3 to 1.0% by mass in the rhizome of American Mayapple (Podophyllum peltatum).[2][3] Another common source of podophyllotoxin is the rhizomes of Podophyllum hexandrum Royle (Berberidaceae).
It is synthesized biologically from two molecules of coniferyl alcohol by phenolic oxidative coupling and a series of oxidations, reductions and methylations.[2]
Structural characteristic
The structure of podophyllotoxin was first elucidated in the 1930s.[4] Podophyllotoxin bears a four consecutive chiral centers, labelled C-1 through C-4. The molecule also contains four almost planar fused rings. Four ends of podophyllotoxin have oxygen atoms at the functional groups dioxoles, methoxys, lactone, and secondary alcohol. [5]

Derivatives of podophyllotoxin are synthesized as properties of the rings and carbon 1 through 4 are diversified. For example, ring A is not essential to antimitotic activity. Aromatization of ring C leads to loss of activity, possibly from ring E no longer being placed on the axial position. In addition, the stereochemistry at C-2 and C-3 configures a trans-lactone, which has more activity than the cis counterpart. Chirality at C-1 is also important as it implies an axial position for ring E. [5]
Biosynthesis
Although the biosynthetic route of podophyllotoxin has yet to be completely elucidated, several studies have suggested a common pathway starting from coniferyl alcohol being converted to (+)-pinoresinol in the presence of a one-electron oxidant [6] through dimerization of stereospecific radical intermediate. Pinoresinol is subsequently reduced in the presence of co-factor NADPH to first lariciresinol, and ultimately secoisolariciresinol. Lactonization on secoisolariciresinol gives rise to matairesinol. Secoisolariciresinol is assumed to be converted to yatein through appropriate quinomethane intermediates [6], leading to podophyllotoxin.

Side effects
Application can be immediately followed by burning or itching. Small sores, itching and peeling skin can also follow. Ingestion or entering the blood stream in small amounts through the dermis can cause fatal poisoning, symptoms include shortness of breath, rapid heart rate and shivers. Most people using the gel do not report these side effects.
Usages and applications
Podophyllotoxin displays a range of activities such as cathartic, purgative, antiviral, vesicant, and antihelminthic. Additionally, the lignan and its derivatives are exciting leads for anti-tumor agent. For instance, podophyllotoxin is the pharmacological precursor for the important anticancer drug etoposide.[6][7]
It is also used as a gel or solution to treat genital warts with noticeably shorter duration and fewer side effects.[8]
Mechanism of action
Podophyllotoxin's anticancer property can be attributed to the inhibition of tubulin polymerization. As podophyllotoxin binds to the tubulin, microtubule formation is prevented. Consequently, podophyllotoxin arrests the cell cycle in the metaphase[2].
Podophyllotoxin derivatives display binding activity to the enzyme topoisomerase II during the late S and early G2 stage. For instance, etoposide binds and stabilizes the temporary break caused by the enzyme, disrupts the reparation of the break through which the double-stranded DNA passes, and consequently stops DNA unwinding and replication[2].
References
- ^ Xu, H; Lv, M; Tian,X (2009). "A review on hemisynthesis, biosynthesis, biological activities, mode of action, and structure-activity relationship of podophyllotoxins: 2003-2007.". Current Medicinal Chemistry 16 (3): 327–349. doi:10.2174/092986709787002682. PMID 19149581.
- ^ a b c d Canel, C; Moraes, RM; Dayan, FE; Ferreira, D (2000). "Molecules of Interest: Podophyllotoxin". Phytochemistry 54 (2): 115–120.
- ^ J. L. Hartwell, A. W. Schrecker (1951). "Components of Podophyllin. V. The Constitution of Podophyllotoxin". Journal of the American Chemical Society 73 (6): 2909–2916. doi:10.1021/ja01150a143.
- ^ Borsche, W.; Niemann J. (1932). "Über Podophyllin". Justus Liebigs Ann. Chem. 494: 126–142. doi:10.1002/jlac.19324940113.
- ^ a b You, Y (2005). "Podophyllotoxin derivatives: current synthetic approaches for new anticancer agents.". Current Pharmaceutical Design 11 (13): 1695–1717. doi:10.2174/1381612053764724. PMID 15892669.
- ^ a b c Gordaliza M, García PA, del Corral JM, Castro MA, Gómez-Zurita MA (2004). "Podophyllotoxin: distribution, sources, applications and new cytotoxic derivatives". Toxicon 44 (4): 441–59. doi:10.1016/j.toxicon.2004.05.008. PMID 15302526. http://linkinghub.elsevier.com/retrieve/pii/S0041010104001953.
- ^ Damayanthi Y, Lown JW (June 1998). "Podophyllotoxins: current status and recent developments". Curr. Med. Chem. 5 (3): 205–52. PMID 9562603.
- ^ Xie FM, Zeng K, Chen ZL, et al. (2007). "[Treatment of recurrent condyloma acuminatum with solid lipid nanoparticle gel containing podophyllotoxin: a randomized double-blinded, controlled clinical trial]" (in Chinese). Nan Fang Yi Ke Da Xue Xue Bao 27 (5): 657–9. PMID 17545082.
Antibiotics and chemotherapeutics for dermatological use (D06) Antibiotics OthersChemotherapeutics Aciclovir • Penciclovir • Idoxuridine • Edoxudine
Imiquimod/Resiquimod • Podophyllotoxin
Docosanol • Tromantadine • Inosine • Lysozyme • IbacitabineOtherDNA virus antivirals (primarily J05, also S01AD and D06BB) Baltimore I DNA-synthesis
inhibitorTK activatedguanine (Aciclovir#/Valacyclovir, Ganciclovir/Valganciclovir, Penciclovir/Famciclovir)
adenine (Vidarabine)Not TK activatedOtherHepatitis B (VII) Nucleoside analogues/NARTIs: Entecavir • Lamivudine • Telbivudine • Clevudine
Nucleotide analogues/NtRTIs: Adefovir • TenofovirMultiple/general Nucleic acid inhibitorsMultiple/unknownErbB2/PI3K PathwayNOV-205§ • NOV-002†Lignans Arctigenin | Globoidnan A | Macelignan | Matairesinol | Pinoresinol | Podophyllotoxin | Secoisolariciresinol | Sesamin | SteganacinLignan glycosides Mammalian lignans (enterolignans) Enterodiol | Enterolactone | Lariciresinol | Hydroxymatairesinol | SyringaresinolNeolignans Flavonolignans Categories:- Antivirals
- Lignans
- Phenol ethers
- Lactones
Wikimedia Foundation. 2010.
