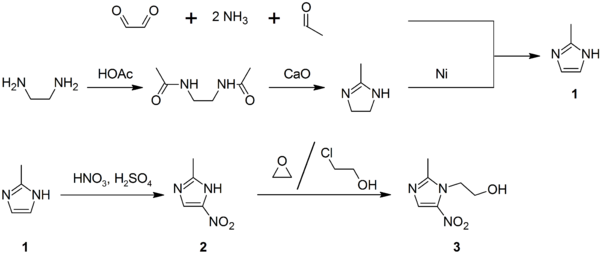- Metronidazole
-
Metronidazole 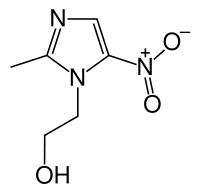
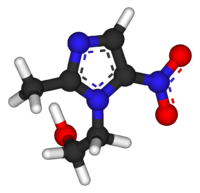
Systematic (IUPAC) name 2-(2-methyl-5-nitro-1H-imidazol-1-yl)ethanol Clinical data Trade names Flagyl AHFS/Drugs.com monograph Pregnancy cat. B(US) B2 (Au) Legal status Prescription Only (S4) (AU) POM (UK) ℞-only (US) Routes oral, topical, rectal, IV, vaginal Pharmacokinetic data Bioavailability 100% (oral)
59–94% (rectal)Metabolism Hepatic Half-life 6–7 hours Excretion Renal (60-80%), biliary (6–15%) Identifiers CAS number 443-48-1 
ATC code A01AB17 , D06BX01, G01AF01, J01XD01, P01AB01, QP51AA01 PubChem CID 4173 DrugBank DB00916 ChemSpider 4029 
UNII 140QMO216E 
KEGG D00409 
ChEBI CHEBI:6909 
ChEMBL CHEMBL137 
Chemical data Formula C6H9N3O3 Mol. mass 171.15 g/mol Physical data Melt. point 159–163 °C (318–325 °F)  (what is this?) (verify)
(what is this?) (verify)Metronidazole (INN) (
 /mɛtrəˈnaɪdəzoʊl/) is a nitroimidazole antibiotic medication used particularly for anaerobic bacteria and protozoa. Metronidazole is an antibiotic, amebicide, and antiprotozoal.[1] It is the drug of choice for first episodes of mild-to-moderate Clostridium difficile infection.[2] It is marketed by Pfizer under the trade name Flagyl in the US, by Sanofi-Aventis globally under the same tradename Flagyl, in Pakistan and Bangladesh it is also available with the brand name of Nidagyl manufactured and marketed by Star Laboratories. In Thailand it is marketed as Mepagyl by Thai Nakhorn Patana. They are also marketed in UK by Milpharm Limited and Almus Pharmaceuticals. Metronidazole was developed in 1960.
/mɛtrəˈnaɪdəzoʊl/) is a nitroimidazole antibiotic medication used particularly for anaerobic bacteria and protozoa. Metronidazole is an antibiotic, amebicide, and antiprotozoal.[1] It is the drug of choice for first episodes of mild-to-moderate Clostridium difficile infection.[2] It is marketed by Pfizer under the trade name Flagyl in the US, by Sanofi-Aventis globally under the same tradename Flagyl, in Pakistan and Bangladesh it is also available with the brand name of Nidagyl manufactured and marketed by Star Laboratories. In Thailand it is marketed as Mepagyl by Thai Nakhorn Patana. They are also marketed in UK by Milpharm Limited and Almus Pharmaceuticals. Metronidazole was developed in 1960.Metronidazole is used also as a gel preparation in the treatment of the dermatological conditions such as rosacea (Rozex and MetroGel by Galderma) and fungating tumours (Anabact, Cambridge Healthcare Supplies).
Contents
Medical uses
Metronidazole is indicated for the treatment of:
Bacterial
- Bacterial vaginosis, commonly associated with overgrowth of Gardnerella species and coinfective anaerobes (Mobiluncus, Bacteroides), in symptomatic patients
- Pelvic inflammatory disease in conjunction with other antibiotics such as ofloxacin, levofloxacin, or ceftriaxone
- Anaerobic infections such as Bacteroides fragilis, spp, Fusobacterium spp, Clostridium spp, Peptostreptococcus spp, Prevotella spp, or any other anaerobes in intra-abdominal abscess, peritonitis, diverticulitis, empyema, pneumonia, aspiration pneumonia, lung abscess, diabetic foot ulcer, meningitis and brain abscesses, bone and joint infections, septicemia, endometritis, or endocarditis
- Helicobacter pylori eradication therapy, as part of a multi-drug regimen in peptic ulcer disease
- Dental infection of bacterial origin, such as periapical abscess, periodontal abscess, acute pericoronitis of impacted or partially erupted teeth; often used in conjunction with Amoxicillin
Protozoal
- Amoebiasis: Infections caused by Entamoeba histolytica.[1]
- Giardiasis: infection of the small intestine caused by the ingestion of infective cysts of protozoan Giardia lamblia.[1]
- Trichomoniasis: infection caused by Trichomonas vaginalis, which is a common cause of vaginitis and is the most frequently presenting new infection of the common sexually transmitted diseases.[1]
Nonspecific
- Prophylaxis for those undergoing potentially contaminated colorectal surgery or appendectomies and may be combined with neomycin[citation needed]
- Crohn's disease with colonic or perianal involvement (non-FDA approved) – believed to be more effective in combination with ciprofloxacin[citation needed]
- Topical metronidazole is indicated for the treatment of rosacea, and in the treatment of malodorous fungating wounds.[3]
Preterm births
Metronidazole has also been used in women to prevent preterm birth associated with bacterial vaginosis, amongst other risk factors including the presence of cervicovaginal fetal fibronectin (fFN). A randomised controlled trial demonstrated that metronidazole was ineffective in preventing preterm delivery in high-risk pregnant women and, conversely, the incidence of preterm delivery was actually higher in women treated with metronidazole.[4]
In a study it has been found that metronidazole is not the right antibiotic to administer in these circumstances and that it was often administered too late to be of use. Clindamycin administered early in the second trimester to women who test positive for bacterial vaginosis seemed to be more effective.[5]
Veterinary use
Metronidazole is not labeled for animal use but is widely used to treat infections of Giardia in dogs, cats, and other companion animals, although it does not reliably clear infection with this organism and is being supplanted by fenbendazole for this purpose in dogs and cats.[6] Metronidazole or simply "Metro" is used in the aquarium hobby to treat ornamental fish as a wide spectrum treatment for bacterial and protozoan infections. It is also used to treat human enteric (gi) and systemic infections. The U.S. Food and Drug Administration (FDA) prohibits the use of metronidazole in food animals.[7]
Adverse effects
Common adverse drug reactions (≥1% of patients) associated with systemic metronidazole therapy include: nausea, diarrhea, and/or metallic taste in the mouth. Intravenous administration is commonly associated with thrombophlebitis. Infrequent adverse effects include: hypersensitivity reactions (rash, itch, flushing, fever), headache, dizziness, vomiting, glossitis, stomatitis, dark urine, and/or paraesthesia.[3]
High doses and/or long-term systemic treatment with metronidazole is associated with the development of leukopenia, neutropenia, increased risk of peripheral neuropathy and/or CNS toxicity.[3]
Metronidazole is listed by the US National Toxicology Program (NTP) as reasonably anticipated to be a human carcinogen. Although some of the testing methods have been questioned[citation needed], oral exposure has been shown to cause cancer in experimental animals.[8] The relationship between exposure to metronidazole and human cancer is unclear.[8] One study (Beard et al. 1988) found an excess in lung cancer among women (even after adjusting for smoking), while other studies (IARC 1987; Thapa et al. 1998) found either no increased risk, or a statistically insignificant risk.[8] [9] It appears to have a fairly low potential for cancer risk and under most circumstances the benefits of treatment outweigh the risk. Metronidazole is listed as a possible carcinogen according to the WHO International Agency for Research on Cancer (IARC).[10]
Due to its potential carcinogenic properties, metronidazole is banned in the EU and the USA for veterinary use in the feed of animals and is banned for use in any food animals in the USA.[11][12] In the USA, this type of restriction is covered under the Delaney clause.
Earlier studies suggested a relation between metronidazole and various birth defects. Those studies are now considered flawed and more recent studies "do not support a significant increased risk for birth defects or other adverse effects on the fetus."[13]
Common adverse drug reactions associated with topical metronidazole therapy include local redness, dryness, and/or skin irritation; and eye watering (if applied near eyes).[3]
Metronidazole toxicity of the brain
Toxic levels of metronidazole can cause symmetrical lesions in the brain in the corpus callosum and dentate nuclei. Metronidazole toxicity is rare (though the actual incidence is not known with certainty). Patients present with nausea, vomiting, dysarthria, vertigo, and confusion. Other side effects of the metronidazole can include dry mouth, diarrhea, headache, dizziness, or peripheral neuropathy. An examination of a patient reveals that the patient is confused and has dysarthria (difficult or unclear articulation of speech that is otherwise linguistically normal), ataxia (loss of full control of bodily movements), abnormal eye movements including nystagmus and ophthalmoparesis. Magnetic resonance imaging (MRI) most often shows bilateral symmetric fluid-attenuated inversion recovery (FLAIR) hyperintense lesions of the dentate nuclei (which is one of the deep cerebellar nuclei), as well as symmetric lesions of the corpus callosum and basal ganglia. The brain lesions seen on the MRI rarely enhance and may be Diffusion-Weighted Imaging (DWI) hyperintense. It has a subacute to acute course. Most reports have been seen in patients who receive approximately one gram a day of metronidazole for over 30 days. [14][citation needed]
Metronidazole can rarely cause central nervous system toxicity; it does not seem to be a dose- or duration-related phenomenon. Most patients will have MRI abnormalities. Prognosis is excellent with metronidazole cessation.[15][16]
Interaction with alcohol
Consuming ethanol (alcohol) while using metronidazole has long been thought to have a disulfiram-like reaction with effects that can include nausea, vomiting, flushing of the skin, tachycardia (accelerated heart rate), and shortness of breath,[17] however there are studies calling that notion into question.[18] Consumption of alcohol should be avoided by patients during systemic metronidazole therapy and for at least 48 hours after completion of treatment.[3] However, the mechanism of this reaction in the clinical setting has recently been questioned by some authors,[19][20] and a possible central toxic serotonin reaction for the alcohol intolerance suggested.[21]
Stevens-Johnson syndrome with mebendazole
Metronidazole alone rarely causes Stevens-Johnson syndrome but is reported to occur at high rates when combined with mebendazole.[22]
Potentially fatal serotonin syndrome
It is important to note that serotonin syndrome is not fully understood. The complex drug interaction can happen after a couple days or take up to months. The exact mechanism is not known, a theory of serotonin dysfunction helps explain how the syndrome presents and how it is to be treated. Signs and symptoms are muscle rigidity, headache, elevated blood pressure, and changes in blood chemistry. The only direct treatment is to discontinue the offending drugs. Recently, there have been reported cases of SSRI/SNRI antidepressant drugs and metronidazole induced serotonin syndrome,[21][23] this information is not included on the metronidazole patient information leaflet. SSRI and SNRI antidepressants include Prozac, Lexapro, Celexa, Zoloft, Effexor, Cymbalta, etc.
Shape and color
Metronidazole is available with a prescription under the brand names Flagyl and Protostat. Other brand or generic formulations may also be available.[24]
Mechanism of action
Metronidazole, taken up by diffusion, is selectively absorbed by anaerobic bacteria and sensitive protozoa. Once taken up by anaerobes, it is non-enzymatically reduced by reacting with reduced ferredoxin, which is generated by pyruvate oxido-reductase. Many of the reduced nitroso intermediates will form sulfinamides and thioether linkages with cystein bearing enzymes deactivating these critical enzymes. As many as 150 separate enzymes are affected.
In addition or alternatively, the metronidazole metabolites are taken up into bacterial DNA, and form unstable molecules. This function only occurs when metronidazole is partially reduced, and because this reduction usually happens only in anaerobic cells, it has relatively little effect upon human cells or aerobic bacteria.[25]
Synthesis
2-Methylimidazole (1) may be prepared via the Debus-Radziszewski imidazole synthesis, or from ethylenediamine and acetic acid, followed by treatment with lime, then Raney nickel. 2-Methylimidazole nitrated to give 2-methyl-4(5)-nitroimidazole (2), which is in turn alkylated with ethylene oxide or 2-chloroethanol to give metronidazole (3):[26][27][28]
References
- ^ a b c d "Metronidazole monograph". drugs.com. http://www.drugs.com/monograph/metronidazole.html.
- ^ Cohen, S. H.; Gerding, D. N.; Johnson, S.; Kelly, C. P.; Loo, V. G.; McDonald, L. C.; Pepin, J.; Wilcox, M. H. et al. (2010). "Clinical Practice Guidelines for Clostridium difficile Infection in Adults: 2010 Update by the Society for Healthcare Epidemiology of America (SHEA) and the Infectious Diseases Society of America (IDSA)". Infection Control and Hospital Epidemiology 31 (5): 431–455. doi:10.1086/651706. PMID 20307191.
- ^ a b c d e Rossi, Simone, ed (2006). Australian Medicines Handbook 2006. Adelaide: Australian Medicines Handbook Pty. ISBN 978-0-9757919-2-9. OCLC 224831213.[page needed]
- ^ Shennan, A.; Crawshaw, S.; Briley, A.; Hawken, J.; Seed, P.; Jones, G.; Poston, L. (2005). "General obstetrics: A randomised controlled trial of metronidazole for the prevention of preterm birth in women positive for cervicovaginal fetal fibronectin: The PREMET Study". BJOG: an International Journal of Obstetrics & Gynaecology 113 (1): 65–74. doi:10.1111/j.1471-0528.2005.00788.x. PMID 16398774.
- ^ Lamont, R. F. (2005). "Can antibiotics prevent preterm birth-the pro and con debate". BJOG: an International Journal of Obstetrics & Gynaecology 112: 67–73. doi:10.1111/j.1471-0528.2005.00589.x. PMID 15715599.
- ^ Barr, S. C.; Bowman, D. D.; Heller, R. L. (1994). "Efficacy of fenbendazole against giardiasis in dogs". American journal of veterinary research 55 (7): 988–990. PMID 7978640.
- ^ Plumb, Donald C. (2008). Veterinary Drug Handbook (6 ed.). Wiley, John & Sons. ISBN 0813820561.
- ^ a b c "Metronidazole CAS No. 443-48-1" (pdf). Report on Carcinogens, Twelvth Edition (2011). U.S. Department of Health and Human Services, Public Health Service, National Toxicology Program. http://ntp.niehs.nih.gov/ntp/roc/twelfth/profiles/Metronidazole.pdf. Retrieved 2011-10-28.
- ^ "Flagyl 375 U.S. Prescribing Information" (pdf). Pfizer. http://www.pfizer.com/pfizer/download/uspi_flagyl_375.pdf.
- ^ "Agents Classified by the IARC Monographs, Volumes 1–100" (PHP). International Agency for Research on Cancer (IARC). World Health Organization. May 2010. http://monographs.iarc.fr/ENG/Classification/index.php. Retrieved 2010-06-06.
- ^ "Metronidazole Summary Report EMEA/MRL/173/96-FINAL" (pdf). Committee for Medicinal Products for Veterinary Use (CVMP). European Medicines Agency. July 1997. http://www.emea.europa.eu/pdfs/vet/mrls/017396en.pdf. Retrieved 2009-12-11.
- ^ "FDA's prohibited drug list". Food Animal Residue Avoidance and Depletion Program (FARAD). USA Food and Drug Administration. 2010. http://www.farad.org/eldu/prohibit.html. Retrieved 2010-06-06.
- ^ "Metronidazole (Flagyl®) and Pregnancy" (pdf). OTIS pregnancy. http://www.otispregnancy.org/files/metronidazole.pdf.
- ^ Aaron Lord. Department of Neurology, Columbia University. Case Report.
- ^ Kuriyama, A.; Jackson, J. L.; Doi, A.; Kamiya, T. (2011). "Metronidazole-Induced Central Nervous System Toxicity". Clinical Neuropharmacology: 1. doi:10.1097/WNF.0b013e3182334b35. PMID 21996645.
- ^ Sinha, S.; Mahadevan, A.; Bindu, P. S.; Taly, A. B.; Chacko, J.; Pramod, K.; Saini, J.; Bharath, R. D. et al. (2011). "Clinical, neuroimaging and pathological features of 5-nitroimidazole-induced encephalo-neuropathy in two patients: Insights into possible pathogenesis". Neurology India 59 (5): 743–747. doi:10.4103/0028-3886.86552. PMID 22019662.
- ^ Cina, S. J.; Russell, R. A.; Conradi, S. E. (1996). "Sudden death due to metronidazole/ethanol interaction". The American journal of forensic medicine and pathology 17 (4): 343–346. PMID 8947362.
- ^ Gupta, N. K.; Woodley, C. L.; Fried, R. (1970). "Effect of metronidazole on liver alcohol dehydrogenase". Biochemical pharmacology 19 (10): 2805–2808. PMID 4320226.
- ^ Williams CS, Woodcock KR (2000). "Do ethanol and metronidazole interact to produce a disulfiram-like reaction?". The Annals of Pharmacotherapy 34 (2): 255–7. doi:10.1345/aph.19118. PMID 10676835. "the authors of all the reports presumed the metronidazole-ethanol reaction to be an established pharmacologic fact. None provided evidence that could justify their conclusions"
- ^ Visapää, J. P.; Tillonen, J. S.; Kaihovaara, P. S.; Salaspuro, M. P. (2002). "Lack of disulfiram-like reaction with metronidazole and ethanol". The Annals of pharmacotherapy 36 (6): 971–974. PMID 12022894.
- ^ a b Karamanakos, P.; Pappas, P.; Boumba, V.; Thomas, C.; Malamas, M.; Vougiouklakis, T.; Marselos, M. (2007). "Pharmaceutical Agents Known to Produce Disulfiram-Like Reaction: Effects on Hepatic Ethanol Metabolism and Brain Monoamines". International Journal of Toxicology 26 (5): 423–432. doi:10.1080/10915810701583010. PMID 17963129.
- ^ Chen, K. T.; Twu, S. J.; Chang, H. J.; Lin, R. S. (2003). "Outbreak of Stevens-Johnson Syndrome/Toxic Epidermal Necrolysis Associated with Mebendazole and Metronidazole Use Among Filipino Laborers in Taiwan". American journal of public health 93 (3): 489–492. PMC 1447769. PMID 12604501. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=1447769.
- ^ Karamanakos, P. N. (2008). "The possibility of serotonin syndrome brought about by the use of metronidazole". Minerva anestesiologica 74 (11): 679. PMID 18971895.
- ^ "PLIVA 334 imprint (metronidazole 500 mg)". drugs.com. http://www.drugs.com/imprints/pliva-334-6108.html. Retrieved 2011-07-04.
- ^ Eisenstein, Barry I.; Schaechter, Moselio (2007). "DNA and Chromosome Mechanics". In Schaechter, Moselio; Engleberg, N. Cary; DiRita, Victor J. et al.. Schaechter's mechanisms of microbial disease. Hagerstown, MD: Lippincott Williams & Wilkins. p. 28. ISBN 978-0-7817-5342-5. http://books.google.com/books?id=1Zl70SLDU3oC&pg=PA28.
- ^ Klaus Ebel, Hermann Koehler, Armin O. Gamer, Rudolf Jäckh (2005), "Imidazole and Derivatives", Ullmann's Encyclopedia of Industrial Chemistry, Weinheim: Wiley-VCH, doi:10.1002/14356007.a13_661
- ^ Paul Actor, Alfred W. Chow, Frank J. Dutko, Mark A. McKinlay (2005), "Chemotherapeutics", Ullmann's Encyclopedia of Industrial Chemistry, Weinheim: Wiley-VCH, doi:10.1002/14356007.a06_173
- ^ Kraft, M. Ya.; Kochergin, P. M.; Tsyganova, A. M.; Shlikhunova, V. S. (1989). "Synthesis of metronidazole from ethylenediamine". Pharmaceutical Chemistry Journal 23 (10): 861. doi:10.1007/BF00764821.
External links
- "Metronidazole for veterinary use". Wedgewood pharmacy. http://www.wedgewoodpharmacy.com/monographs/metronidazole.asp.
- "Metronidazole". Merck manuals. http://www.merck.com/mmpe/lexicomp/metronidazole.html.
- "Metronidazole". patient.co.uk. http://www.patient.co.uk/showdoc/30003224/.
- "Metronidazole". Drug Information Portal. U.S. National Library of Medicine. http://druginfo.nlm.nih.gov/drugportal/dpdirect.jsp?name=Metronidazole.
Stomatological preparations (A01) Caries prophylactic agents Anti-infectives and antiseptics Amphotericin B • Benzoxonium chloride • Chlorhexidine • Chlortetracycline • Clotrimazole • Domiphen bromide • Doxycycline • Eugenol • Hexetidine • Hydrogen peroxide • Mepartricin • Metronidazole • Miconazole • Minocycline • Natamycin • Neomycin • Oxyquinoline • Polynoxylin • Sodium perborate • Tetracycline • Tibezonium iodideCorticosteroids (Glucocorticoids) Other Antibiotics and chemotherapeutics for dermatological use (D06) Antibiotics OthersChemotherapeutics Aciclovir • Penciclovir • Idoxuridine • Edoxudine
Imiquimod/Resiquimod • Podophyllotoxin
Docosanol • Tromantadine • Inosine • Lysozyme • IbacitabineOtherMetronidazoleGynecological anti-infectives and antiseptics (G01) Antibiotics Arsenic compounds Quinoline derivatives Organic acids Sulfonamides SulfatolamideImidazole derivatives Metronidazole • Clotrimazole • Miconazole • Econazole • Ornidazole • Isoconazole • Tioconazole • Ketoconazole • Fenticonazole • Azanidazole • Propenidazole • Butoconazole • Omoconazole • Oxiconazole • FlutrimazoleTriazole derivatives Other Clodantoin • Inosine • Policresulen • Nifuratel • Furazolidone • Methylrosaniline • Povidone-iodine • Ciclopirox • Protiofate • Lactobacillus fermentum • Copper usnateAntibacterials: nucleic acid inhibitors (J01E, J01M) Antifolates
(inhibits
purine metabolism,
thereby inhibiting
DNA and RNA synthesis)Other/ungroupedCombinationsTopoisomerase
inhibitors/
quinolones/
(inhibits
DNA replication)1st g.2nd g.Ciprofloxacin# • Enoxacin‡ • Fleroxacin‡ • Lomefloxacin • Nadifloxacin • Ofloxacin • Norfloxacin • Pefloxacin • Rufloxacin3rd g.4th g.Besifloxacin • Clinafloxacin† • Garenoxacin • Gemifloxacin • Moxifloxacin • Gatifloxacin‡ • Sitafloxacin • Trovafloxacin‡/Alatrofloxacin‡ • PrulifloxacinVet.Related (DG)Anaerobic DNA
inhibitorsNitrofuran derivativesRNA synthesis #WHO-EM. ‡Withdrawn from market. Clinical trials: †Phase III. §Never to phase III Antiparasitics – antiprotozoal agents – Chromalveolate antiparasitics (P01) Alveo-
lateIndividual
agentsOtherSulfadoxine • sulfamethoxypyrazineCoformulationFansidar# (sulfadoxine/pyrimethamine)OtherCombi-
nationsartemether-lumefantrine#
artesunate-amodiaquine (ASAQ)
artesunate-mefloquine (ASMQ)
dihydroartemisinin-piperaquine
artesunate-pyronaridineOther combinations
(not co-formulated)artesunate/SP • artesunate/mefloquine •
quinine/tetracycline • quinine/doxycycline • quinine/clindamycinHetero-
kont#WHO-EM. ‡Withdrawn from market. Clinical trials: †Phase III. §Never to phase III Antiparasitics – antiprotozoal agents – Excavata antiparasitics (P01) Discicristata TrypanosomiasisAfrican trypanosomiasis: ornithine (Eflornithine#) • arsenical (Melarsoprol#) • benzamidine (Pentamidine#) • naphthalenesulfonate (Suramin#)
Chagas disease: nitroimidazole (Benznidazole#) • nitrofuran (Nifurtimox#)Trichozoa nitroimidazole (Metronidazole#, Tinidazole) • benzimidazole (Albendazole)
thiazole (Nitazoxanide) • nitrofuran (Furazolidone)
aminoacridine (Quinacrine)Trichomoniasisnitroimidazole (Metronidazole, Secnidazole)
oxyquinoline (Iodoquinol) • tetracycline (Doxycycline) • neomycin (Paromomycin)#WHO-EM. ‡Withdrawn from market. Clinical trials: †Phase III. §Never to phase III Antiparasitics – antiprotozoal agents – agents against amoebozoa/amebicide (P01) Entamoeba Nitroimidazole derivativesOtherHydroxyquinoline derivativesCl (Chlorquinaldol) • Br (Tilbroquinol, Broxyquinoline) • I (Diiodohydroxyquinoline) • I,Cl (Clioquinol)
related: ChiniofonDichloroacetamide derivativesOther/ungroupedarsenic (Arsthinol, Difetarsone, Glycobiarsol) • phenanthroline (Phanquinone) • aminoacridine (Mepacrine) • quinazoline (Trimetrexate) • thiazole (Tenonitrozole) • sesquiterpene (Fumagillin)Acanthamoeba #WHO-EM. ‡Withdrawn from market. Clinical trials: †Phase III. §Never to phase III
Wikimedia Foundation. 2010.