- Rifabutin
-
Rifabutin 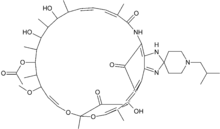
Systematic (IUPAC) name (9S,12E,14S,15R,16S,17R,18R,19R,20S,
21S,22E,24Z)-6,16,18,20-tetrahydroxy-1'-
isobutyl-14-methoxy-7,9,15,17,19,21,25-
hepta-methyl-spiro[9,4-(epoxypentadeca
[1,11,13]trienimino)-2H-furo-[2',3':7,8]-naphth
[1,2-d]imidazol-2,4'-piperidin]-5,10,26-(3H,9H)-
trione-16-acetateClinical data Trade names Mycobutin AHFS/Drugs.com monograph MedlinePlus a693009 Pregnancy cat. C (Australia), B (United States) Legal status POM (UK), ℞-only (U.S.) Routes Oral Pharmacokinetic data Bioavailability 85% Protein binding 85% Metabolism Hepatic Half-life 28 to 62 hours (mean) Excretion Renal and fecal Identifiers CAS number 72559-06-9 
ATC code J04AB04 PubChem CID 6323490 DrugBank APRD00094 ChemSpider 10482168 
UNII 1W306TDA6S 
KEGG D00424 
ChEBI CHEBI:45367 
ChEMBL CHEMBL444633 
Chemical data Formula C46H62N4O11 Mol. mass 847.005 g/mol SMILES eMolecules & PubChem - InChI=1S/C46H62N4O11/c1-22(2)21-50-18-16-46(17-19-50)48-34-31-32-39(54)28(8)42-33(31)43(56)45(10,61-42)59-20-15-30(58-11)25(5)41(60-29(9)51)27(7)38(53)26(6)37(52)23(3)13-12-14-24(4)44(57)47-36(40(32)55)35(34)49-46/h12-15,20,22-23,25-27,30,37-38,41,49,52-54H,16-19,21H2,1-11H3,(H,47,57)/b13-12+,20-15+,24-14-/t23-,25+,26+,27+,30-,37-,38+,41+,45-/m0/s1

Key:ATEBXHFBFRCZMA-VXTBVIBXSA-N
 (what is this?) (verify)
(what is this?) (verify)Rifabutin (Rfb) is a bactericidal antibiotic drug primarily used in the treatment of tuberculosis. The drug is a semi-synthetic derivative of rifamycin S. Its effect is based on blocking the DNA-dependent RNA-polymerase of the bacteria. It is effective against Gram-positive and some Gram-negative bacteria, but also against the highly resistant Mycobacteria, e.g. Mycobacterium tuberculosis, M. leprae and M. avium intracellulare.
Contents
History
Scientists at the Italian drug company Achifar discovered rifabutin in 1975. Eventually Archifar became part of Farmitalia Carlo Erba, a unit of the conglomerate Montedison. This company's Adria Laboratories subsidiary filed for Food and Drug Administration (FDA) approval of rifabutin under the brand name Mycobutin in the early 1990s and the drug gained FDA approval in December 1992.
Rifabutin (RFB) is primarily bactericidal antibiotic drug used to treat tuberculosis. Its effect on bacteria is based on DNA-dependent RNA polymerase blocking drug rifamycin S., a semi-synthetic derivative, efficient for example, in highly resistant mycobacteria, the Gram-positive and some are effective against Gram-negative bacteria, but also against Mycobacterium tuberculosis, M. and M. leprae avium intracellulare.
Indications
Rifabutin is now recommended as first-line treatment for tuberculosis.[1] Rifampicin is more widely used because of its cheaper cost.
Rifabutin is used in the treatment of mycobacterium avium complex disease, a bacterial infection most commonly encountered in late-stage AIDS patients.
Rifabutin is well tolerated in patients with HIV-related tuberculosis (TB), but patients with low CD4 cell counts have a high risk of treatment failure or relapse due to acquired rifamycin resistance, a new study found.
Since patients co-infected with TB and HIV / AIDS are likely to get TB treated first, doctors and patients should be aware of a possible rifamycin resistance issue, if the CD4 is so suppressed at the time TB treatment is to begin.
Rifabutin is also used in trials for treating Crohn's Disease as part of the anti-MAP therapy. In a Phase 3 study it could not prove a significant role of MAP in Crohn's disease.[2]
Its main usefulness lies in the fact that it has lesser drug interactions than rifampicin therefore HIV infected patients on HAART are given rifabutin for treatment of TB
It has also found to be useful in the treatment of (Chlamydia) Chlamydophila pneumoniae (Cpn) Infection. See external link.
Supply
.
References
- ^ Guidelines for the programmatic management of drug-resistant tuberculosis: emergency update 2008 (WHO/HTM/TB/2008.402). Geneva, Switzerland: World Health Organization. 2008. p. ix. ISBN 978 92 4 154758 1. http://www.who.int/tb/publications/2008/programmatic_guidelines_for_mdrtb/en/index.html.
- ^ . doi:10.1053/j.gastro.2007.03.031. PMID 17570206.
External links
Antimycobacterials, including tuberculosis treatment and leprostatic agents (J04) Nucleic acid inhibitor Rifampicin# • Rifabutin • RifapentineAntifolates/DSIASA4-Aminosalicylic acid# (Calcium aminosalicylate, Sodium aminosalicylate)Protein synthesis inhibitor Cell envelope antibiotic Peptidoglycan layerAlanine analogue: Cycloserine#Arabinogalactan layerEthylenediamine/arabinosyltransferase inhibitor: Ethambutol#
SQ109†Mycolic acid layerHydrazides/mycolic acid synth. inhibition: Isoniazid#
Thiocarbamides: Ethionamide# • Prothionamide • ThiocarlideOther/unknown phenazine (Clofazimine)# • pyrazine (Pyrazinamide#, Morinamide) • isoxazole (Terizidone) • R207910/TMC207†Combinations Rifampicin/isoniazid/pyrazinamide#WHO-EM. ‡Withdrawn from market. Clinical trials: †Phase III. §Never to phase III Antibacterials: nucleic acid inhibitors (J01E, J01M) Antifolates
(inhibits
purine metabolism,
thereby inhibiting
DNA and RNA synthesis)Sulfonamides
(DHPS inhibitor)Other/ungroupedCombinationsTopoisomerase
inhibitors/
quinolones/
(inhibits
DNA replication)1st g.2nd g.Ciprofloxacin# • Enoxacin‡ • Fleroxacin‡ • Lomefloxacin • Nadifloxacin • Ofloxacin • Norfloxacin • Pefloxacin • Rufloxacin3rd g.4th g.Besifloxacin • Clinafloxacin† • Garenoxacin • Gemifloxacin • Moxifloxacin • Gatifloxacin‡ • Sitafloxacin • Trovafloxacin‡/Alatrofloxacin‡ • PrulifloxacinVet.Related (DG)Anaerobic DNA
inhibitorsNitrofuran derivativesRNA synthesis #WHO-EM. ‡Withdrawn from market. Clinical trials: †Phase III. §Never to phase III Categories:- Rifamycin antibiotics
- InChI=1S/C46H62N4O11/c1-22(2)21-50-18-16-46(17-19-50)48-34-31-32-39(54)28(8)42-33(31)43(56)45(10,61-42)59-20-15-30(58-11)25(5)41(60-29(9)51)27(7)38(53)26(6)37(52)23(3)13-12-14-24(4)44(57)47-36(40(32)55)35(34)49-46/h12-15,20,22-23,25-27,30,37-38,41,49,52-54H,16-19,21H2,1-11H3,(H,47,57)/b13-12+,20-15+,24-14-/t23-,25+,26+,27+,30-,37-,38+,41+,45-/m0/s1
Wikimedia Foundation. 2010.
