- Nitroimidazole
-
4-Nitroimidazole[1]  5-Nitro-1H-imidazole
5-Nitro-1H-imidazoleIdentifiers CAS number 3034-38-6 PubChem 18208 ChemSpider 10637918 
Jmol-3D images Image 1 - c1cn(cn1)[N+](=O)[O-]
Properties Molecular formula C3H3N3O2 Molar mass 113.07 g/mol Melting point 303 °C (dec.)
Hazards R-phrases R20/21/22 R36/37/38 S-phrases S26 S36/37 Main hazards Xn  (verify) (what is:
(verify) (what is:  /
/ ?)
?)
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references 4-Nitroimidazole is an imidazole derivative that contains a nitro group.
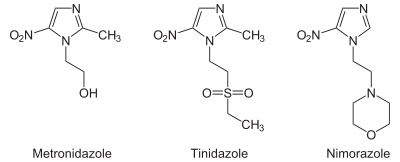
Several derivatives of nitroimidazole constitute the class of nitroimidazole antibiotics that have been used to combat anaerobic bacterial and parasitic infections.[2] Perhaps the most common example is metronidazole (Flagyl). Other heterocycles such as nitrothiazoles (thiazole) are also used for this purpose. Nitroheterocycles may be reductively activated in hypoxic cells, and then undergo redox recycling or decompose to toxic products.[3]
References
- ^ 4-Nitroimidazole at Sigma-Aldrich
- ^ Mital A (2009). "Synthetic Nitroimidazoles: Biological Activities and Mutagenicity Relationships". Sci Pharm 77 (3): 497–520. doi:10.3797/scipharm.0907-14.
- ^ Juchau, MR (1989). "Bioactivation in chemical teratogenesis". Annu. Rev. Pharmacol. Toxicol. 29: 165–167. doi:10.1146/annurev.pa.29.040189.001121. PMID 2658769.
Antiparasitics – antiprotozoal agents – Excavata antiparasitics (P01) Discicristata TrypanosomiasisAfrican trypanosomiasis: ornithine (Eflornithine#) • arsenical (Melarsoprol#) • benzamidine (Pentamidine#) • naphthalenesulfonate (Suramin#)
Chagas disease: nitroimidazole (Benznidazole#) • nitrofuran (Nifurtimox#)Trichozoa nitroimidazole (Metronidazole#, Tinidazole) • benzimidazole (Albendazole)
thiazole (Nitazoxanide) • nitrofuran (Furazolidone)
aminoacridine (Quinacrine)Trichomoniasisnitroimidazole (Metronidazole, Secnidazole)
oxyquinoline (Iodoquinol) • tetracycline (Doxycycline) • neomycin (Paromomycin)#WHO-EM. ‡Withdrawn from market. Clinical trials: †Phase III. §Never to phase III Antiparasitics – antiprotozoal agents – agents against amoebozoa/amebicide (P01) Entamoeba Nitroimidazole derivativesOtherHydroxyquinoline derivativesCl (Chlorquinaldol) • Br (Tilbroquinol, Broxyquinoline) • I (Diiodohydroxyquinoline) • I,Cl (Clioquinol)
related: ChiniofonDichloroacetamide derivativesOther/ungroupedarsenic (Arsthinol, Difetarsone, Glycobiarsol) • phenanthroline (Phanquinone) • aminoacridine (Mepacrine) • quinazoline (Trimetrexate) • thiazole (Tenonitrozole) • sesquiterpene (Fumagillin)Acanthamoeba #WHO-EM. ‡Withdrawn from market. Clinical trials: †Phase III. §Never to phase III Antibacterials: nucleic acid inhibitors (J01E, J01M) Antifolates
(inhibits
purine metabolism,
thereby inhibiting
DNA and RNA synthesis)Other/ungroupedCombinationsTopoisomerase
inhibitors/
quinolones/
(inhibits
DNA replication)1st g.2nd g.Ciprofloxacin# • Enoxacin‡ • Fleroxacin‡ • Lomefloxacin • Nadifloxacin • Ofloxacin • Norfloxacin • Pefloxacin • Rufloxacin3rd g.4th g.Besifloxacin • Clinafloxacin† • Garenoxacin • Gemifloxacin • Moxifloxacin • Gatifloxacin‡ • Sitafloxacin • Trovafloxacin‡/Alatrofloxacin‡ • PrulifloxacinVet.Related (DG)Anaerobic DNA
inhibitorsNitrofuran derivativesRNA synthesis #WHO-EM. ‡Withdrawn from market. Clinical trials: †Phase III. §Never to phase III Categories:- Nitroimidazoles
Wikimedia Foundation. 2010.