- Trimethoprim
-
Trimethoprim 

Systematic (IUPAC) name 5-(3,4,5- trimethoxybenzyl) pyrimidine- 2,4- diamine Clinical data AHFS/Drugs.com monograph MedlinePlus a684025 Pregnancy cat. B3(AU) C(US) Legal status Prescription Only (S4) (AU) POM (UK) Routes Oral Pharmacokinetic data Bioavailability 90–100% Metabolism hepatic Half-life 8–10 hours Excretion renal 50–60% Identifiers CAS number 738-70-5 
ATC code J01EA01 QJ51EA01 PubChem CID 5578 DrugBank DB00440 ChemSpider 5376 
UNII AN164J8Y0X 
KEGG D00145 
ChEBI CHEBI:45924 
ChEMBL CHEMBL22 
Chemical data Formula C14H18N4O3 Mol. mass 290.32 g/mol SMILES eMolecules & PubChem  (what is this?) (verify)
(what is this?) (verify)Trimethoprim (INN) (
 /traɪˈmɛθəprɪm/) is a bacteriostatic antibiotic mainly used in the prophylaxis and treatment of urinary tract infections.
/traɪˈmɛθəprɪm/) is a bacteriostatic antibiotic mainly used in the prophylaxis and treatment of urinary tract infections.It belongs to the class of chemotherapeutic agents known as dihydrofolate reductase inhibitors. Trimethoprim was formerly marketed by GlaxoSmithKline under trade names including Proloprim, Monotrim and Triprim; but these trade names have been licensed to various generic pharmaceutical manufacturers. In clinical use it is often abbreviated TRI or TMP; its common laboratory abbreviation is W.
Contents
Mechanism of action
 Tetrahydrofolate synthesis pathway
Tetrahydrofolate synthesis pathwayTrimethoprim acts by interfering with the action of bacterial dihydrofolate reductase, inhibiting synthesis of tetrahydrofolic acid. Tetrahydrofolic acid is an essential precursor in the de novo synthesis of the intermediate Thymidine monophosphate (dTMP), precursor of DNA metabolite Thymidine triphosphate.[1] Bacteria are unable to take up folic acid from the environment (i.e. the infection host) and are thus dependent on their own de novo synthesis. Inhibition of the enzyme starves the bacteria of nucleotides necessary for DNA replication causing, in certain circumstances, cell lethality due to thymineless death. This drug was developed by George H. Hitchings and collaborators, who shared the Nobel Prize for Physiology or Medicine in 1988 for the discovery of antifolates.[2]
Co-trimoxazole
Trimethoprim was commonly (from 1969 to 1980 in the UK) used in a 1:5 combination with sulfamethoxazole, a sulfonamide antibiotic, which inhibits an earlier step in the folate synthesis pathway (see diagram below). This combination, also known as co-trimoxazole, TMP-sulfa, or TMP-SMX, results in an in vitro synergistic antibacterial effect by inhibiting successive steps in folate synthesis. This claimed benefit was not seen in general clinical use.[3][4]
The combinations use has been declining due to reports of sulfamethoxazole bone marrow toxicity, resistance and lack of greater efficacy in treating common urine and chest infections,[5][6][7][8] and side effects of antibacterial sulfonamides. As a consequence, the use of co-trimoxazole was restricted in 1995 [9] following the availability of trimethoprim (not in combination) in 1980.
With its greater efficacy against a limited number of bacteria, Co-trimoxazole remains indicated for some infections; for example, it is used as prophylaxis in patients at risk for Pneumocystis jirovecii pneumonia (e.g. AIDS patients and those with some hematological malignancies) and as therapy in Whipple's disease. Gram positive bacteria are generally or moderately susceptible.
Clinical indications
Trimethoprim, used as monotherapy (since 1980 in the UK), is indicated for the prophylaxis and treatment of urinary tract infections. (Co-trimoxazole, with its greater efficacy against a limited number of bacteria, and parasites remains indicated for some infections, see above.)
Contraindications and reactions
Trimethoprim can cause thrombocytopenia (low levels of platelets) by lowering folic acid levels; this may also cause megaloblastic anemia. Trimethoprim antagonises the epithelial sodium channel (ENaC) in the distal tubule, thus acting like amiloride, this can cause hyperkalemia. Trimethoprim also competes with creatinine for secretion into the renal tubule, this can cause an artefactual rise in the serum creatinine. Use in EHEC infections may lead to an increase in expression of Shiga toxin.[10] Due to the fact that it crosses the placenta and can affect folate metabolism, trimethoprim is relatively contraindicated during pregnancy, especially the first trimester.[11] It may be involved in a reaction similar to disulfiram when alcohol is consumed after it is used, particularly when used in combination with sulfamethoxazole.[12][13]
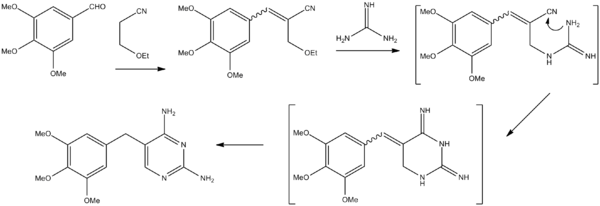
Chemistry
Stenbuck, P.; Hood, H. M.; 1962, U.S. Patent 3,049,544.
References
- ^ de novo synthesis pathway for dTTP
- ^ http://nobelprize.org/nobel_prizes/medicine/laureates/1988/press.html
- ^ Brumfitt W, Hamilton-Miller JM (December 1993). "Reassessment of the rationale for the combinations of sulphonamides with diaminopyrimidines". J Chemother 5 (6): 465–9. PMID 8195839.
- ^ Brumfitt W, Hamilton-Miller JM (February 1993). "Limitations of and indications for the use of co-trimoxazole". J Chemother 6 (1): 3–11. PMID 8071675.
- ^ Bean DC, Livermore DM, Papa I, Hall LM (November 2005). "Resistance among Escherichia coli to sulphonamides and other antimicrobials now little used in man". J Antimicrob Chemother 56 (5): 962–4. doi:10.1093/jac/dki332. PMID 16150859. http://jac.oxfordjournals.org/cgi/content/full/56/5/962.
- ^ Felmingham D, Reinert RR, Hirakata Y, Rodloff A (September 2002). "Increasing prevalence of antimicrobial resistance among isolates of Streptococcus pneumoniae from the PROTEKT surveillance study, and compatative in vitro activity of the ketolide, telithromycin". J Antimicrob Chemother 50 (Suppl S1): 25–37. doi:10.1093/jac/dkf808 (inactive 2010-03-20). PMID 12239226. http://jac.oxfordjournals.org/cgi/reprint/50/suppl_2/25.
- ^ Johnson JR, Manges AR, O'Bryan TT, Riley LW (June 29 2002). "A disseminated multidrug-resistant clonal group of uropathogenic Escherichia coli in pyelonephritis". Lancet 359 (9325): 2249–51. doi:10.1016/S0140-6736(02)09264-4. PMID 12103291.
- ^ Lawrenson RA, Logie JW (December 2001). "Antibiotic failure in the treatment of urinary tract infections in young women". J Antimicrob Chemother 48 (6): 895–901. doi:10.1093/jac/48.6.895. PMID 11733475. - suggest some small advantage in UTIs
- ^ "Co-trimoxazole use restricted". Drug Ther Bull 33 (12): 92–3. December 1995. doi:10.1136/dtb.1995.331292. PMID 8777892.
- ^ http://www.cdc.gov/ncidod/EID/vol6no5/kimmett.htm
- ^ "Trimethoprim". netdoctor.co.uk.
- ^ Edwards DL, Fink PC, Van Dyke PO (February 17, 1986). "Disulfiram-like reaction associated with intravenous trimethoprim-sulfamethoxazole and metronidazole". J Clinical pharmacy 5 (12): 999. http://cat.inist.fr/?aModele=afficheN&cpsidt=8287529.
- ^ Heelon MW, White M (February 17, 1998). "Disulfiram-cotrimoxazole reaction". J Pharmacotherapy 18 (4): 869. http://cat.inist.fr/?aModele=afficheN&cpsidt=2340043.
External links
- Nucleic acid inhibitors (PDF file).
Antibacterials: nucleic acid inhibitors (J01E, J01M) Antifolates
(inhibits
purine metabolism,
thereby inhibiting
DNA and RNA synthesis)Other/ungroupedCombinationsTopoisomerase
inhibitors/
quinolones/
(inhibits
DNA replication)1st g.2nd g.Ciprofloxacin# • Enoxacin‡ • Fleroxacin‡ • Lomefloxacin • Nadifloxacin • Ofloxacin • Norfloxacin • Pefloxacin • Rufloxacin3rd g.4th g.Besifloxacin • Clinafloxacin† • Garenoxacin • Gemifloxacin • Moxifloxacin • Gatifloxacin‡ • Sitafloxacin • Trovafloxacin‡/Alatrofloxacin‡ • PrulifloxacinVet.Related (DG)Anaerobic DNA
inhibitorsNitrofuran derivativesRNA synthesis Categories:- Antibiotics
- Dihydrofolate reductase inhibitors
- Pyrimidines
- World Health Organization essential medicines
- Phenol ethers
- Aromatic amines
Wikimedia Foundation. 2010.