- Nitrofurantoin
-
Nitrofurantoin 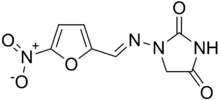
Systematic (IUPAC) name (E)-1-[(5-nitro-2-furyl)methylideneamino]imidazolidine-2,4-dione Clinical data AHFS/Drugs.com monograph MedlinePlus a682291 Pregnancy cat. B Legal status Rx, PoM Routes oral, rectal[1] Pharmacokinetic data Bioavailability 40% Metabolism liver (75%) Half-life 20 minutes Excretion urine and bile Identifiers CAS number 67-20-9 
ATC code J01XE01 PubChem CID 6604200 DrugBank APRD00191 ChemSpider 5036498 
UNII 927AH8112L 
KEGG D00439 
ChEMBL CHEMBL572 
Chemical data Formula C8H6N4O5 Mol. mass 238.16 SMILES eMolecules & PubChem  (what is this?) (verify)
(what is this?) (verify)Nitrofurantoin is an antibiotic which is marketed under the following brand names; Furadantin, Macrobid, Macrodantin, Nitrofur Mac, Nitro Macro, Nifty-SR, Martifur-MR, Martifur-100 (in India), Urantoin, and Uvamin (in Middle East). It is usually used in treating urinary tract infection. Like many other drugs, it is often used against E. coli.
Contents
Use
Resistance to other antibiotics has led to increased interest in this agent.[2]
It is sometimes described as being appropriate to use in pregnant patients[3] (along with other agents such as sulfisoxazole or cephalexin).[4] This is in contrast to agents such as trimethoprim and ciprofloxacin which may not be appropriate for pregnant women.
Pharmacology
Organisms are said to be susceptible to nitrofurantoin if their minimum inhibitory concentration (MIC) is 32 μg/mL or less. The peak blood concentration of nitrofurantoin following an oral dose of nitrofurantoin 100 mg, is less than 1 μg/mL and may be undetectable; tissue penetration is negligible; the drug is well concentrated in the urine: 75% of the dose is rapidly metabolised by the liver, but 25% of the dose is excreted in the urine unchanged, reliably achieving levels of 200 μg/ml or more. For this reason, nitrofurantoin cannot be used to treat anything other than simple cystitis.
At the concentrations achieved in urine, nitrofurantoin is bacteriocidal.
Nitrofurantoin and the quinolone antibiotics are mutually antagonistic in vitro. It is not known whether this is of clinical significance, but the combination should be avoided.
Resistance to nitrofurantoin may be chromosomal or plasmid mediated and involves inhibition of nitrofuran reductase.[5] Acquired resistance in E. coli continues to be rare.
Nitrofurantoin and its metabolites are excreted mainly by the kidneys. In renal impairment, the concentration achieved in urine may be subtherapeutic. Nitrofurantoin should not be used in patients with a creatinine clearance of 60 mL/min or less. However a retrospective chart review may suggest that nitrofurantoin is not contraindicated in this population.[6]
Mechanism
The mechanism of action of nitrofurantoin is unique and complex. The drug works by damaging bacterial DNA, since its reduced form is highly reactive. This is made possible by the rapid reduction of nitrofurantoin inside the bacterial cell by flavoproteins (nitrofuran reductase) to multiple reactive intermediates that attack ribosomal proteins, DNA,[7] respiration, pyruvate metabolism and other macromolecules within the cell. It is not known which of the actions of nitrofurantoin is primarily responsible for its bactericidal activity.
Uses
The normal adult dose of nitrofurantoin is 50 to 100 mg four times daily for seven days. If a long-acting preparation (e.g., Macrobid) is used then the dose is 100 mg twice daily. The pediatric dose is 5–7 mg/kg/day in four divided doses.[8] or when in 25 mg/5ml oral suspension then pediatric dose is 3 mg/kg/day in four divided doses.[9] Nitrofurantoin should be taken with food, as this improves the absorption of the drug by 45%.
Nitrofurantoin is only clinically proven for use against E. coli or Staph. saprophyticus. It may also have in vitro activity against:
- Coagulase-negative staphylococci
- Enterococcus faecalis,
- Staphylococcus aureus,
- Streptococcus agalactiae,
- Citrobacter species,
- Klebsiella species,
and is used in the treatment of infections caused by these organisms. Only a minority of Enterobacter species and Klebsiella species are sensitive to nitrofurantoin; nitrofurantoin has no activity against
- Acinetobacter species,
- Morganella species,
- Proteus species,
- Providentia species,
- Serratia species, or
- Pseudomonas species.
Nitrofurantoin must never be used to treat pyelonephritis,[10] renal abscess, and pyeloempyema because of extremely poor tissue penetration and low blood levels. Urinary catheter infections may be treated with nitrofurantoin if there are no systemic features; the catheter must be changed after 48 hours of antibiotics and treatment is ineffective if the catheter is not replaced or removed.
Adverse effects
Nitrofurantoin can cause nausea and vomiting, fever, rash, hypersensitivity pneumonitis.[citation needed] It can also cause pulmonary fibrosis.[11] All these side effects are much more common in the elderly.
Patients should be informed that nitrofurantoin colours urine a dark orange-brown; this is completely harmless.
Neonates (babies up to the age of one month) have immature enzyme systems in their red blood cells (glutathione instability) and nitrofurantoin must therefore not be used because it can cause haemolytic anaemia. For the same reason, nitrofurantoin should not be given to pregnant women after 38 weeks of pregnancy, or who are about to give birth.
Nitrofurantoin is contraindicated in patients with decreased renal function (CrCl < 60mL/min) due to systemic accumulation and subtherapeutic levels reached in the urinary tract. However a retrospective chart review may suggest that nitrofurantoin is not contraindicated in this population.[6]
Use in food
Residues from the breakdown of nitrofuran veterinary antibiotics, including nitrofurantoin, have been found in chicken in Vietnam, China, Brazil, and Thailand.[12] The European Union banned the use of nitrofurans in food producing animals by classifying it in ANNEX IV (list of pharmacologically active substances for which no maximum residue limits can be fixed) of the Council Regulation 2377/90. The Food and Drug Administration (FDA) of the United States has prohibited furaltadone since February 1985 and withdrew the approval for the other nitrofuran drugs (except some topical uses) in January 1992. The topical use of furazolidone and nitrofurazone was prohibited in 2002. Australia prohibited the use of nitrofurans in food production in 1992. Japan did not allocate MRLs for nitrofurans leading to the implementation of a "zero tolerance or no residue standard". In Thailand, the Ministry of Health issued in 2001 Proclamation No. 231 MRL of veterinary drug in food which did not allocate MRL for nitrofurans. The Ministry of Agriculture and Cooperatives had already prohibited importation and use of furazolidone and nitrofurazone in animal feed in 1999 which was extended to all nitrofurans in 2002. Several metabolites of nitrofurans, such as furazolidone, furaltadone and nitrofurazone cause cancer or genetic damage in rats.[12]
Precautions
Nitrofurantoin must be taken with food and in rare cases can cause bleeding in the stomach, vomiting and other gastrointestinal disruptions if these warnings are not adhered to. Nitrofurantoin is contraindicated in patients with glucose-6-phosphate dehydrogenase deficiency because of risk of extravascular hemolysis resulting in anemia.
Trade names
- Furadantin (U.S., UK)
- Macrobid (long acting preparation for twice daily dosing available in U.S., Canada, and UK)
- Macrodantin (U.S., UK)
- Furatin, Niftran(by Ranbaxy), Niftas(by Intas Pharmaceuticals) (India)
- Furanit
- Uvamin (Middle East)
References
- ^ Parrott, E. L.; Matheson Jr, L. E. (1977). "Rectal absorption of nitrofurantoin". Journal of Pharmaceutical Sciences 66 (7): 955–958. doi:10.1002/jps.2600660713. PMID 886458.
- ^ Garau J (January 2008). "Other antimicrobials of interest in the era of extended-spectrum beta-lactamases: fosfomycin, nitrofurantoin and tigecycline". Clin. Microbiol. Infect. 14 Suppl 1: 198–202. doi:10.1111/j.1469-0691.2007.01852.x. PMID 18154548. http://www3.interscience.wiley.com/resolve/openurl?genre=article&sid=nlm:pubmed&issn=1198-743X&date=2008&volume=14&issue=&spage=198.
- ^ Lee M, Bozzo P, Einarson A, Koren G (June 2008). "Urinary tract infections in pregnancy". Can Fam Physician 54 (6): 853–4. PMC 2426978. PMID 18556490. http://www.cfp.ca/cgi/pmidlookup?view=long&pmid=18556490.
- ^ "Urinary Tract Infections During Pregnancy - February 1, 2000 - American Academy of Family Physicians". http://www.aafp.org/afp/20000201/713.html.
- ^ McCalla DR, Kaiser C, Green MHL (1978). "Genetics of nitrofurazone resistance in Escherichia coli". J Bacteriol 133: 10–16.
- ^ a b Bains A, Buna D, Hoag NA (2009). "A retrospective review assessing the efficacy and safety of nitrofurantoin in renal impairment". Canadian Pharmacists Journal 142: 248–252. doi:10.3821/1913-701X-142.5.248. http://www.cpjournal.ca/perlserv/?request=get-document&doi=10.3821%2F1913-701X-142.5.248.
- ^ Tu Y, McCalla DR (1975). "Effect of activated nitrofurans on DNA,". Biochem Biophys Acta 402: 142–49.
- ^ http://www.umm.edu/altmed/drugs/nitrofurantoin-092200.htm
- ^ http://www.medicines.org.uk/EMC/medicine/23895/SPC/Furadantin+25mg+5ml+Oral+Suspension+OR+Nitrofurantoin+25mg+5ml+Oral+Suspension/
- ^ Richards WA, et al. (1955). "Nitrofurantoin: Clinical and laboratory studies in urinary tract infections". Arch Intern Med 96: 437–50. PMID 13257939.
- ^ Goemaere NN, Grijm K, van Hal PT, den Bakker MA (2008). "Nitrofurantoin-induced pulmonary fibrosis: a case report". J Med Case Reports 2: 169. doi:10.1186/1752-1947-2-169. PMC 2408600. PMID 18495029. http://www.jmedicalcasereports.com/content/2//169.
- ^ a b FAO: Nitrofuran study
External links
- drugs.com for Macrodantin
- Meds-Help.com for nitrofurantoin side effects, interactions and information
- RxList for Macrobid (Nitrofurantoin)
Antibacterials: nucleic acid inhibitors (J01E, J01M) Antifolates
(inhibits
purine metabolism,
thereby inhibiting
DNA and RNA synthesis)Other/ungroupedCombinationsTopoisomerase
inhibitors/
quinolones/
(inhibits
DNA replication)1st g.2nd g.Ciprofloxacin# • Enoxacin‡ • Fleroxacin‡ • Lomefloxacin • Nadifloxacin • Ofloxacin • Norfloxacin • Pefloxacin • Rufloxacin3rd g.4th g.Besifloxacin • Clinafloxacin† • Garenoxacin • Gemifloxacin • Moxifloxacin • Gatifloxacin‡ • Sitafloxacin • Trovafloxacin‡/Alatrofloxacin‡ • PrulifloxacinVet.Related (DG)Anaerobic DNA
inhibitorsNitrofuran derivativesRNA synthesis Categories:- Antibiotics
- Hydantoins
- World Health Organization essential medicines
- Nitrofurans
Wikimedia Foundation. 2010.
