- Nalidixic acid
-
Nalidixic acid 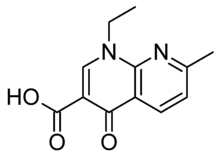
Systematic (IUPAC) name 1-ethyl-7-methyl-4-oxo-[1,8]naphthyridine-3-carboxylic acid Clinical data AHFS/Drugs.com Consumer Drug Information Pregnancy cat. B U.S. Legal status ? Routes Oral Pharmacokinetic data Protein binding 90% Metabolism Partially Hepatic Half-life 6-7 hours, significantly longer in renal impairment Identifiers CAS number 389-08-2 
ATC code J01MB02 PubChem CID 4421 DrugBank APRD01133 ChemSpider 4268 
UNII 3B91HWA56M 
KEGG D00183 
ChEBI CHEBI:100147 
ChEMBL CHEMBL5 
Chemical data Formula C12H12N2O3 Mol. mass 232.235 g/mol SMILES eMolecules & PubChem  (what is this?) acid (verify)
(what is this?) acid (verify)Nalidixic acid (tradenames Nevigramon, Neggram, Wintomylon and WIN 18,320) is the first of the synthetic quinolone antibiotics. In the technical sense, it is a naphthyridone, not a quinolone: its ring structure is a 1,8-naphthyridines nucleus that contains two nitrogen atoms, unlike quinoline, which has a single nitrogen atom.[1]
Synthetic quinolone antibiotics were discovered by George Lesher and coworkers as a byproduct of chloroquine manufacture in the 1960s.[1]
Nalidixic acid is effective against both gram-positive and gram-negative bacteria. In lower concentrations, it acts in a bacteriostatic manner; that is, it inhibits growth and reproduction. In higher concentrations, it is bactericidal, meaning that it kills bacteria instead of merely inhibiting their growth.
It is especially used in treating urinary tract infections, caused, for example, by Escherichia coli, Proteus, Shigella, Enterobacter, and Klebsiella.. It is also a tool in studies as a regulation of bacterial division. It selectively and reversibly blocks DNA replication in susceptible bacteria. Nalidixic acid and related antibiotics inhibit a subunit of DNA gyrase and induce formation of relaxation complex analogue. It also inhibits the nicking dosing activity on the subunit of DNA gyrase that releases the positive binding stress on the supercoiled DNA. It is the only FDA approved quinolone for treating UTI infections in children (3).
Contents
Adverse effects
Convulsions and hyperglycaemia[2]
See also
- Fluoroquinolone toxicity
- Fluoroquinolone
- DNA gyrase
References
- ^ a b Emmerson, A.; Jones, A. (2003). "The quinolones: decades of development and use". The Journal of antimicrobial chemotherapy 51 Suppl 1 (90001): 13–20. doi:10.1093/jac/dkg208. PMID 12702699.
- ^ Fraser AG, Harrower AD (December 1977). "Convulsions and hyperglycaemia associated with nalidixic acid". Br Med J 2 (6101): 1518. doi:10.1136/bmj.2.6101.1518. PMC 1632822. PMID 589309. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=1632822.
3 Barkley, Nghiem, (Sept. 2011) "AAP reviews
use of systemic and topical Quinolones" Medscape educational briefs
External links
Antibacterials: nucleic acid inhibitors (J01E, J01M) Antifolates
(inhibits
purine metabolism,
thereby inhibiting
DNA and RNA synthesis)Sulfonamides
(DHPS inhibitor)Other/ungroupedCombinationsTopoisomerase
inhibitors/
quinolones/
(inhibits
DNA replication)1st g.Cinoxacin‡ • Flumequine • Nalidixic acid • Oxolinic acid • Pipemidic acid • Piromidic acid • Rosoxacin2nd g.Ciprofloxacin# • Enoxacin‡ • Fleroxacin‡ • Lomefloxacin • Nadifloxacin • Ofloxacin • Norfloxacin • Pefloxacin • Rufloxacin3rd g.4th g.Besifloxacin • Clinafloxacin† • Garenoxacin • Gemifloxacin • Moxifloxacin • Gatifloxacin‡ • Sitafloxacin • Trovafloxacin‡/Alatrofloxacin‡ • PrulifloxacinVet.Related (DG)Anaerobic DNA
inhibitorsNitrofuran derivativesRNA synthesis Categories:- Quinolone antibiotics
- 1,8 Naphthyridines
- WIN compounds
Wikimedia Foundation. 2010.
