- Sulfanilamide
-
Sulfanilamide 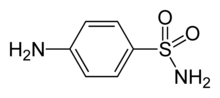
Systematic (IUPAC) name 4-aminobenzenesulfonamide Clinical data AHFS/Drugs.com Consumer Drug Information Pregnancy cat. ? Legal status ? Identifiers CAS number 63-74-1 
ATC code J01EB06 D06BA05 QJ01EQ06 PubChem CID 5333 DrugBank APRD00438 ChemSpider 5142 
UNII 21240MF57M 
KEGG D08543 
ChEBI CHEBI:45373 
ChEMBL CHEMBL21 
Chemical data Formula C6H8N2O2S Mol. mass 172.20 g/mol SMILES eMolecules & PubChem Physical data Density 1.08 g/cm³ Melt. point 165 °C (329 °F)  (what is this?) (verify)
(what is this?) (verify)Sulfanilamide (also spelled sulphanilamide) is a sulfonamide antibacterial. Chemically, it is a molecule containing the sulfonamide functional group attached to an aniline. As a sulfonamide antibiotic, it functions by competitively inhibiting (i.e., by acting as a substrate analogue) enzymatic reactions involving para-aminobenzoic acid (PABA).[1] PABA is needed in enzymatic reactions that produce folic acid which acts as a coenzyme in the synthesis of purine, pyrimidine and other amino acids.
The term "sulfanilamides" is also used to describe a family of molecules containing these functional groups. Examples include:
Gerhard Domagk, who directed the testing of the prodrug Prontosil in 1935,[2] and Jacques and Thérèse Tréfouël, who along with Federico Nitti and Daniel Bovet in the laboratory of Ernest Fourneau at the Pasteur Institute, discovered the active component, 4-aminobenzenesulfonamide,[3] are generally credited with the discovery of sulfanilamide as a chemotherapeutic agent. Domagk was awarded the Nobel Prize for his work.[4]
See also
- Sulfonamide (medicine)
- Nazi human experimentation -- Sulfonamide experiments
- Elixir sulfanilamide
External links
References
- ^ Michael Kent, Advanced Biology, Oxford University Press, 2000, p. 46 ISBN 9780199141951
- ^ G. Domagk, "Ein Beitrag zur Chemotherapie der bakteriellen Infektionen", Deutsche Medizinische Wochenschrift, 61, feb. 15, 1935, p. 250.
- ^ J. et T. Tréfouël, F. Nitti and D. Bovet, "Activité du p-aminophénylsulfamide sur l’infection streptococcique expérimentale de la souris et du lapin", C. R. Soc. Biol., 120, nov. 23, 1935, p. 756.
- ^ (French) Daniel Bovet, "Les étapes de la découverte de la sulfamidochrysoïdine dans les laboratoires de recherche de la firme Bayer à Wuppertal-Elberfeld (1927-1932)", in Une chimie qui guérit : Histoire de la découverte des sulfamides, Coll. "Médecine et Société", Payot, Paris, 1988, p. 307.
Antibiotics and chemotherapeutics for dermatological use (D06) Antibiotics Tetracycline and derivativesOthersChemotherapeutics Aciclovir • Penciclovir • Idoxuridine • Edoxudine
Imiquimod/Resiquimod • Podophyllotoxin
Docosanol • Tromantadine • Inosine • Lysozyme • IbacitabineOtherAntibacterials: nucleic acid inhibitors (J01E, J01M) Antifolates
(inhibits
purine metabolism,
thereby inhibiting
DNA and RNA synthesis)Sulfonamides
(DHPS inhibitor)Sulfaisodimidine • Sulfamethizole • Sulfadimidine • Sulfapyridine • Sulfafurazole • Sulfanilamide (Prontosil) • Sulfathiazole • SulfathioureaOther/ungroupedsulfanilamide (Sulfacetamide, Sulfametrole)CombinationsTopoisomerase
inhibitors/
quinolones/
(inhibits
DNA replication)1st g.2nd g.Ciprofloxacin# • Enoxacin‡ • Fleroxacin‡ • Lomefloxacin • Nadifloxacin • Ofloxacin • Norfloxacin • Pefloxacin • Rufloxacin3rd g.4th g.Besifloxacin • Clinafloxacin† • Garenoxacin • Gemifloxacin • Moxifloxacin • Gatifloxacin‡ • Sitafloxacin • Trovafloxacin‡/Alatrofloxacin‡ • PrulifloxacinVet.Related (DG)Anaerobic DNA
inhibitorsNitrofuran derivativesRNA synthesis Categories:- Sulfonamides
- Chemistry stubs
Wikimedia Foundation. 2010.
