- Gentamicin
-
Gentamicin 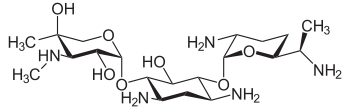

Systematic (IUPAC) name (3R,4R,5R)-2-{[(1S,2S,3R,4S,6R)-4,6-
diamino-3-{[(2R,3R,6S)-
3-amino-6-[(1R)-
1-(methylamino)ethyl]oxan-2-yl]oxy}-
2-hydroxycyclohexyl]oxy}-5-methyl-
4-(methylamino)oxane-3,5-diolClinical data AHFS/Drugs.com monograph MedlinePlus a682275 Pregnancy cat. D Legal status POM (UK) Routes IV, IM, topical Pharmacokinetic data Bioavailability limited oral bioavailability Protein binding 0-10% Half-life 2 hrs Excretion renal Identifiers CAS number 1403-66-3 
ATC code D06AX07 J01GB03 S01AA11 S02AA14 S03AA06 QA07AA91 QG01AA91 QG51AA04 QJ51GB03 PubChem CID 3467 IUPHAR ligand 2427 DrugBank DB00798 ChemSpider 390067 
UNII T6Z9V48IKG 
KEGG D08013 
ChEBI CHEBI:27412 
ChEMBL CHEMBL195892 
Chemical data Formula C21H43N5O7 Mol. mass 477.596 g/mol SMILES eMolecules & PubChem  (what is this?) (verify)
(what is this?) (verify)Gentamicin is an aminoglycoside antibiotic, used to treat many types of bacterial infections, particularly those caused by Gram-negative organisms.[1] However, gentamicin is not used for Neisseria gonorrhoeae, Neisseria meningitidis or Legionella pneumophila. Gentamicin is also ototoxic and nephrotoxic, with this toxicity remaining a major problem in clinical use.[1]
It is synthesized by Micromonospora, a genus of Gram-positive bacteria widely present in the environment (water and soil). To highlight their specific biological origins, gentamicin and other related antibiotics produced by this genus (verdamicin, mutamicin, sisomicin, netilmicin, retymicin) generally have their spellings ending in ~micin and not in ~mycin. Gentamicin is a bactericidal antibiotic that works by binding the 30S subunit of the bacterial ribosome, interrupting protein synthesis.
Like all aminoglycosides, when gentamicin is given orally, it is not systemically active. This is because it is not absorbed to any appreciable extent from the small intestine. It is administered intravenously, intramuscularly or topically to treat infections. It appears to be completely eliminated unchanged in the urine. Urine must be collected for many days to recover all of a given dose because the drug binds avidly to certain tissues.
E. coli has shown some resistance to gentamicin, despite being Gram-negative. Reluctance to use gentamicin for empirical therapy has led to increased use of alternative broad-spectrum antibiotics, which some experts suggest has led to the prevalence of antibiotic-resistant bacterial infections by Golden Staph and other so-called "superbugs".[1]
Gentamicin is one of the few heat-stable antibiotics that remain active even after autoclaving, which makes it particularly useful in the preparation of some microbiological growth media. It is used during orthopaedic surgery when high temperatures are required for the setting of cements (e.g. hip replacements).[2]
Contents
Spectrum of activity
Active against a wide range of human bacterial infections, mostly Gram-negative bacteria including Pseudomonas, Proteus, Serratia, and the Gram-positive Staphylococcus.[3] Gentamicin is not used for Neisseria gonorrhoeae, Neisseria meningitidis or Legionella pneumophila bacterial infections (because of the risk of the patient going into shock from lipid A endotoxin found in certain Gram-negative organisms). Gentamicin is also useful against Yersinia pestis and its relatives.
Side effects
These aminoglycosides are toxic to the sensory cells of the ear, but they vary greatly in their relative effects on hearing versus balance. Gentamicin is a vestibulotoxin, and can cause permanent loss of equilibrioception, caused by damage to the vestibular apparatus of the inner ear, usually if taken at high doses or for prolonged periods of time, but there are well documented cases in which gentamicin completely destroyed the vestibular apparatus after three to five days.[citation needed] A small number of affected individuals have a normally harmless mutation in their mitochondrial RNA (m1555 A>G), that allows the gentamicin to affect their cells. The cells of the ear are particularly sensitive to this, sometimes causing complete hearing loss.[4] However, gentamicin is sometimes used intentionally for this purpose in severe Ménière's disease, to disable the vestibular apparatus.
Gentamicin can also be highly nephrotoxic, particularly if multiple doses accumulate over a course of treatment. For this reason gentamicin is usually dosed by body weight. Various formulae exist for calculating gentamicin dosage. Also trough and peak serum levels of gentamicin are monitored during treatment, generally before and after the third dose is infused.
Gentamicin, like other aminoglycosides, causes nephrotoxicity by inhibiting protein synthesis in renal cells. This mechanism specifically causes necrosis of cells in the proximal tubule, resulting in acute tubular necrosis which can lead to acute renal failure.[5]
Side effects of gentamicin toxicity vary from patient to patient. Side effects may become apparent shortly after or up to months after gentamicin is administered. Symptoms of gentamicin toxicity include:
- Balance difficulty
- Bouncing, unsteady vision
- Ringing in the ears (tinnitus)
- Difficulty multi-tasking, particularly when standing
Psychiatric symptoms related to gentamicin can occur. These include anorexia, confusion, depression, disorientation and visual hallucinations.[6] Immediate professional help should be sought if any of these symptoms or others appear after administration of aminoglycosides. General medical practitioners should refer patients with such symptoms to an otolaryngologist, commonly known as an 'ear, nose, and throat doctor', for comprehensive tests.
A number of factors and determinants should be taken into account when using gentamicin, including differentiation between empirical and directed therapy which will affect dosage and treatment period.[1] Many medical practitioners freely administer gentamicin as an antibiotic without advising patients of the severe and permanent potential ramifications of its use. Gentamicin is well known to be a cheap, low cost yet old medicine as compared to modern alternatives, and is typically US$3–6 per dosage less than modern alternatives.
Many people recover from gentamicin toxicity naturally over time if the drug is discontinued, but they recover slowly and usually incompletely.[citation needed] Sometimes the toxicity of gentamicin can still increase over months after the last dose. Upon cessation of gentamicin therapy symptoms such as tinnitus and imbalance may become less pronounced. Sensori-neural hearing loss caused by gentamicin toxicity is permanent however.
Production and usage in research
Gentamicin is produced by a fermentation procedure. It was discovered by a Chinese microbiologist, Yue Wang. The majority of the world's gentamicin production takes place in China and South Korea; the last European producer is Lek, part of Sandoz group.
Gentamicin has been used since the early 1980s in microbiological research. The gentamicin protection assay enables researchers to quantify the ability of pathogenic bacteria to invade eukaryotic cells. It takes advantage of the fact that gentamicin is not able to penetrate eukaryotic cells.
References
- ^ a b c d Moulds, Robert and Jeyasingham, Melanie (October 2010). "Gentamicin: a great way to start". Australian Prescriber (33): 134–135. http://www.australianprescriber.com/magazine/33/5/134/5.
- ^ Hendriks JGE, van Horn JR, van der Mei HC, and Busscher, HJ (2004). "Backgrounds of antibiotic-loaded bone cement and prosthesis-related infection". Biomaterials 25 (3): 545–556. doi:10.1016/S0142-9612(03)00554-4. PMID 14585704.
- ^ Gentamicin: Drug Information Provided by Lexi-Comp: Merck Manual Professional
- ^ Gentamicin Toxicity at the American Hearing Research Foundation
- ^ Sundin DP, Sandoval R, Molitoris BA: Gentamicin Inhibits Renal Protein and Phospholipid Metabolism in Rats: Implications Involving Intracellular Trafficking. J Am Soc Nephrol 12:114-123, 2001
- ^ AJ Giannini, HR Black. Psychiatric, Psychogenic and Somatopsychic Disorders Handbook. Garden City, NY. Medical Examination Publishing Co.,1978. Pp.136-137. ISBN 0-87488-596-5.
Antibiotics and chemotherapeutics for dermatological use (D06) Antibiotics OthersAminoglycosides: Neomycin • Gentamicin • Amikacin
other: Fusidic acid • Bacitracin • Tyrothricin • MupirocinChemotherapeutics Aciclovir • Penciclovir • Idoxuridine • Edoxudine
Imiquimod/Resiquimod • Podophyllotoxin
Docosanol • Tromantadine • Inosine • Lysozyme • IbacitabineOtherAntibacterials: protein synthesis inhibitors (J01A, J01B, J01F, J01G, QJ01XQ) 30S -mycin (Streptomyces)Neomycin# (Framycetin, Paromomycin, Ribostamycin)
Kanamycin# (Amikacin, Arbekacin, Bekanamycin, Dibekacin, Tobramycin)
Paromomycin-micin (Micromonospora)Tetracyclines50S PleuromutilinsErythromycin# • Azithromycin# • Spiramycin • Midecamycin • Oleandomycin • Roxithromycin • Josamycin • Troleandomycin • Clarithromycin • Miocamycin • Rokitamycin • Dirithromycin • Flurithromycin • Ketolide (Telithromycin, Cethromycin, Solithromycin)EF-G Steroid antibacterialsOtologicals (S02) Anti-infectives Acetic acid • Aluminium acetotartrate • Boric acid • Chloramphenicol • Chlorhexidine • Ciprofloxacin • Clioquinol • Gentamicin • Hydrogen peroxide • Miconazole • Neomycin • Nitrofurazone • Ofloxacin • Polymyxin B • Rifamycin • TetracyclineCorticosteroids Analgesics and anesthetics M: EAR
anat(e/p)/phys/devp
noco/cong, epon
proc, drug(S2)
Categories:- Aminoglycoside antibiotics
- World Health Organization essential medicines
- Toxicology
- Otologicals
Wikimedia Foundation. 2010.
