- Azithromycin
-
Azithromycin 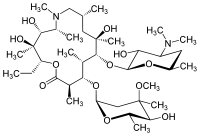
Systematic (IUPAC) name (2R,3S,4R,5R,8R,10R,11R,12S,13S,14R)-2-ethyl-3,4,10-trihydroxy-3,5,6,8,10,12,14-heptamethyl-15-oxo- 11-{[3,4,6-trideoxy-3-(dimethylamino)-β-D-xylo-]oxy}-1-oxa-6-azacyclopentadec-13-yl 2,6-dideoxy-3-C-methyl-3-O-methyl-α-L-ribo-hexopyranoside Clinical data Trade names Zithromax AHFS/Drugs.com monograph MedlinePlus a697037 Licence data US FDA:link Pregnancy cat. B1(AU) B(US) Legal status ℞-only (US) Routes Oral (capsule or suspension), intravenous, ophthalmic Pharmacokinetic data Bioavailability 38% for 250 mg capsules Metabolism Hepatic Half-life 68 hours Excretion Biliary, renal (4.5%) Identifiers CAS number 83905-01-5 
ATC code J01FA10 S01AA26 PubChem CID 55185 DrugBank APRD00397 ChemSpider 10482163 
UNII J2KLZ20U1M 
KEGG D07486 
ChEBI CHEBI:2955 
ChEMBL CHEMBL529 
Synonyms 9-deoxy-9a-aza-9a-methyl-9a-homoerythromycin A Chemical data Formula C38H72N2O12 Mol. mass 748.984 g·mol−1 SMILES eMolecules & PubChem - InChI=1S/C38H72N2O12/c1-15-27-38(10,46)31(42)24(6)40(13)19-20(2)17-36(8,45)33(52-35-29(41)26(39(11)12)16-21(3)48-35)22(4)30(23(5)34(44)50-27)51-28-18-37(9,47-14)32(43)25(7)49-28/h20-33,35,41-43,45-46H,15-19H2,1-14H3/t20-,21-,22+,23-,24-,25+,26+,27-,28+,29-,30+,31-,32+,33-,35+,36-,37-,38-/m1/s1

Key:MQTOSJVFKKJCRP-BICOPXKESA-N
 (what is this?) (verify)
(what is this?) (verify)Azithromycin (Zithromax, and others) is an azalide, a subclass of macrolide antibiotics. Azithromycin is one of the world's best-selling antibiotics.[1] It is derived from erythromycin, with a methyl-substituted nitrogen atom incorporated into the lactone ring, thus making the lactone ring 15-membered.
Azithromycin is used to treat or prevent certain bacterial infections, most often those causing middle ear infections, strep throat, pneumonia, typhoid, and sinusitis. In recent years, it has been used primarily to prevent bacterial infections in infants and those with weaker immune systems. It is also effective against certain sexually transmitted infections, such as non-gonococcal urethritis, chlamydia, and cervicitis. Recent studies have indicated it also to be effective against late-onset asthma, but these findings are controversial and not widely accepted.[2][3]
Contents
Medical uses
Azithromycin is used to treat many different infections including acute otitis media, streptococcal pharyngitis, gastrointestinal infections such as traveler's diarrhea, respiratory tract infections such as pneumonia, cellulitis, babesiosis, bartonella, chancroid cholera, donovanosis, leptospirosis, lyme disease, malaria, mycobacterium avium complex, neisseria meningitis, pelvic inflammatory disease, pertussis, scrub typhus, toxoplasmosis, and salmonella.[4] It is used to prevent bacterial endocarditis and some sexually transmitted illnesses post sexual assault.[4]
It has a similar antimicrobial spectrum as erythromycin, but is more effective against certain Gram-negative bacteria, in particular, Haemophilus influenzae[citation needed]. Azithromycin resistance has been described[5] and is endemic in many areas. It is notably ineffective against MRSA.[citation needed]
Azithromycin has been shown to be effective against malaria when used in combination with artesunate or quinine; the optimal dose for this is not yet known.[6]
Adverse effects
Most common side-effects are gastrointestinal: diarrhea (5%), nausea (3%), abdominal pain (3%), and vomiting. Fewer than 1% of patients stop taking the drug due to side-effects. Nervousness, dermatologic reactions, and anaphylaxis have been reported. As with all antimicrobial agents, pseudomembranous colitis can occur during and up to several weeks after azithromycin therapy. This drug may interfere with the effectiveness of birth control pills; other forms of contraception may be required during the treatment period. Azithromycin suspension has an objectionable taste, so can be difficult to administer to young children, i.e., 2–5 years, who may spit it out.
Occasional patients have developed cholestatic hepatitis or delirium. Accidental intravenous overdosage in an infant caused severe heart block, resulting in residual encephalopathy.[7][8]
Mechanism of action
Azithromycin prevents bacteria from growing by interfering with their protein synthesis. Azithromycin binds to the 50S subunit of the bacterial ribosome, and thus inhibits translation of mRNA. Nucleic acid synthesis is not affected.
Pharmacokinetics
Unlike erythromycin,azithromycin is acid-stable and can therefore be taken orally with no need of protection from gastric acids. It is readily absorbed, but its absorption is greater on an empty stomach. Time to peak concentration in adults is 2.1 to 3.2 hours for oral dosage forms and one to two hours after a dose. Due to the high concentration in phagocytes, azithromycin is actively transported to the site of infection. During active phagocytosis, large concentrations of azithromycin are released. The concentration of azithromycin in the tissues can be over 50 times higher than in plasma.[citation needed] This is due to ion trapping and the high lipid solubility (volume of distribution is too low).
Azithromycin's half-life allows a large single dose to be administered and yet maintain bacteriostatic levels in the infected tissue for several days.
Metabolism
According to Davis' Drug Guide for Nurses, following a single 500 mg dose, the half-life of azithromycin is 11–14 hours. The longer half-life of 68 hours is achieved only when multiple doses are consumed, allowing a "steady state" of medication in the body.
Biliary excretion of azithromycin, predominantly unchanged, is a major route of elimination. Over the course of a week, approximately 6% of the administered dose appears as unchanged drug in urine.
Etymology
Azithromycin's name is derived from the azane-substituent and Erythromycin.
History
A team of researchers at the Croatian pharmaceutical company Pliva — Gabrijela Kobrehel, Gorjana Radobolja-Lazarevski, Zrinka Tamburašev, led by Dr. Slobodan Đokić — discovered azithromycin in 1980. It was patented in 1981. In 1986, Pliva and Pfizer signed a licensing agreement, which gave Pfizer exclusive rights for the sale of azithromycin in Western Europe and the United States. Pliva put its azithromycin on the market in Central and Eastern Europe under the brand name of Sumamed in 1988. Pfizer launched azithromycin under Pliva's licence in other markets under the brand name Zithromax in 1991.
After several years, the U.S. Food and Drug Administration approved AzaSite, an ophthalmic formulation of azithromycin, for the treatment of eye infections. AzaSite is marketed in the U.S. and Canada by Inspire Pharmaceuticals, a wholly owned subsidiary of Merck.[9]
Available forms
Azithromycin is commonly administered in tablet or oral suspension (a one-dose version was made available in 2005). It is also available for intravenous injection and in a 1% ophthalmic solution. Tablets come in doses of 250 mg and 500 mg. Oral suspension comes in strengths of 100 mg/5 mL and 200 mg/5 mL. The 250 mg tablets are often dispensed in packages of six and commonly referred to as a "Z-Pak," whereas the 500 mg tablets are commonly available commercially in a pack of three tablets, or "Tri-Pak," intended as a three-day treatment. A common dose of oral azithromycin therapy consists of a "double dose" of medication on the first day of treatment and subsequent treatment for four or five additional days. With the "Z-Pak," this means two 250 mg tablets (a total of 500 mg) on the first day and one 250 mg tablet once daily for the next four days.
Pfizer brand-name, i.e. Zithromax, azithromycin tablets are mottled pink, unscored, film-coated, modified-oval-shaped tablets containing azithromycin monohydrate and the following inactive ingredients: butylated hydroxytoluene, calcium phosphate, carmine, colloidal silicon dioxide, FD&C red # 40 lake, FD&C yellow # 6 lake, hypromellose (2910, 15cP), lactose monohydrate, magnesium stearate, pregelatinized starch, sodium lauryl sulfate, talc, titanium dioxide, and triacetin.
References
- ^ Azythromycin: A world best-selling antibiotic - Pliva
- ^ Hahn DL (October 1995). "Treatment of Chlamydia pneumoniae infection in adult asthma: a before-after trial". J Fam Pract 41 (4): 345–51. PMID 7561707.
- ^ Klausner JD, Passaro D, Rosenberg J, Thacker WL, Talkington DF, Werner SB, Vugia DJ (January 1998). "Enhanced control of an outbreak of Mycoplasma pneumoniae pneumonia with azithromycin prophylaxis". Journal of Infectious Diseases 177 (1): 161–6. doi:10.1086/513818. PMID 9419183.
- ^ a b "Azithromycin". The American Society of Health-System Pharmacists. http://www.drugs.com/monograph/azithromycin.html. Retrieved 3 April 2011.
- ^ Chisholm SA, Neal TJ, Alawattegama AB, Birley HD, Howe RA, Ison CA (August 2009). "Emergence of high-level azithromycin resistance in Neisseria gonorrhoeae in England and Wales". The Journal of antimicrobial chemotherapy 64 (2): 353–8. doi:10.1093/jac/dkp188. PMID 19468025.
- ^ Noedl H, Krudsood S, Chalermratana K, et al. (November 2006). "Azithromycin combination therapy with artesunate or quinine for the treatment of uncomplicated Plasmodium falciparum malaria in adults: a randomized, phase 2 clinical trial in Thailand". Clinical Infectious Diseases 43 (10): 1264–71. doi:10.1086/508175. PMID 17051490.
- ^ Tilelli JA, Smith KM, Pettignano R. Life-threatening bradyarrhythmia after massive azithromycin overdose. Pharmacotherapy 26: 147-150, 2006.
- ^ R. Baselt, Disposition of Toxic Drugs and Chemicals in Man, 8th edition, Biomedical Publications, Foster City, CA, 2008, pp. 132-133.
- ^ Merck Completes Acquisition of Inspire Pharmaceuticals, Inc.
External links
- "azithromycin" at medicinenet.com
- Azithromycin. Drug Information Portal. United States National Library of Medicine (NLM).
- Azithromycin Side Effects.
Antibacterials: protein synthesis inhibitors (J01A, J01B, J01F, J01G, QJ01XQ) 30S -mycin (Streptomyces)Neomycin# (Framycetin, Paromomycin, Ribostamycin)
Kanamycin# (Amikacin, Arbekacin, Bekanamycin, Dibekacin, Tobramycin)
Paromomycin-micin (Micromonospora)Tetracyclines50S Linezolid • Torezolid • Eperezolid • Posizolid • RadezolidPleuromutilinsRetapamulin • Tiamulin • ValnemulinErythromycin# • Azithromycin# • Spiramycin • Midecamycin • Oleandomycin • Roxithromycin • Josamycin • Troleandomycin • Clarithromycin • Miocamycin • Rokitamycin • Dirithromycin • Flurithromycin • Ketolide (Telithromycin, Cethromycin, Solithromycin)EF-G Steroid antibacterialsCategories:- Antimalarial agents
- Macrolide antibiotics
- Pfizer
- World Health Organization essential medicines
- InChI=1S/C38H72N2O12/c1-15-27-38(10,46)31(42)24(6)40(13)19-20(2)17-36(8,45)33(52-35-29(41)26(39(11)12)16-21(3)48-35)22(4)30(23(5)34(44)50-27)51-28-18-37(9,47-14)32(43)25(7)49-28/h20-33,35,41-43,45-46H,15-19H2,1-14H3/t20-,21-,22+,23-,24-,25+,26+,27-,28+,29-,30+,31-,32+,33-,35+,36-,37-,38-/m1/s1
Wikimedia Foundation. 2010.