- Roxithromycin
-
Roxithromycin 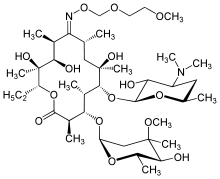
Systematic (IUPAC) name (3R,4S,5S,6R,7R,9R,11S,12R,13S,14R)-6-[(2S,3R,4S,6R)-4-d-3-hydroxy-6-methyloxan-2-yl]oxy-14-ethyl-7,12,13-trihydroxy-4-[(2R,4R,5S,6S)-5-hydroxy-4-methoxy-4,6-dimethyloxan-2-yl]oxy-10-(2-methoxyethoxymethoxyimino)-3,5,7,9,11,13-hexamethyl-1-oxacyclotetradecan-2-one Clinical data AHFS/Drugs.com International Drug Names Pregnancy cat. ? (USA)
B1 (Aus)Legal status ? Pharmacokinetic data Metabolism Liver, peak concentration averaging 2 hours after ingestion. Half-life 12 hours Identifiers CAS number 80214-83-1 ATC code J01 PubChem CID 6915744 DrugBank APRD01305 ChemSpider 5291557 
UNII 21KOF230FA 
KEGG D01710 
ChEBI CHEBI:48935 
ChEMBL CHEMBL1214185 
Chemical data Formula C41H76N2O15 Mol. mass 837.047 g/mol SMILES eMolecules & PubChem - InChI=1S/C41H76N2O15/c1-15-29-41(10,49)34(45)24(4)31(42-53-21-52-17-16-50-13)22(2)19-39(8,48)36(58-38-32(44)28(43(11)12)18-23(3)54-38)25(5)33(26(6)37(47)56-29)57-30-20-40(9,51-14)35(46)27(7)55-30/h22-30,32-36,38,44-46,48-49H,15-21H2,1-14H3/b42-31+/t22-,23-,24+,25+,26-,27+,28+,29-,30+,32-,33+,34-,35+,36-,38+,39-,40-,41-/m1/s1

Key:RXZBMPWDPOLZGW-XMRMVWPWSA-N
 (what is this?) (verify)
(what is this?) (verify)Roxithromycin is a semi-synthetic macrolide antibiotic. It is used to treat respiratory tract, urinary and soft tissue infections. Roxithromycin is derived from erythromycin, containing the same 14-membered lactone ring. However, an N-oxime side chain is attached to the lactone ring. It is also currently undergoing clinical trials for the treatment of male-pattern hair loss.[1]
Roxithromycin is available under several brandnames, for example, Xthrocin, Roxl-150, Roxo, Surlid, Rulide, Biaxsig, Roxar, Roximycin, Roxomycin, Rulid, Tirabicin and Coroxin. Roxithromycin is not available in the United States. Roxithromycin has also been tested to possess antimalarial activity.
Contents
History
German pharmaceutical company Hoechst Uclaf brought out roxithromycin in 1987.
Available forms
Roxithromycin is commonly available as tablets or oral suspension.
Mechanism of action
Roxithromycin prevents bacteria from growing, by interfering with their protein synthesis. Roxithromycin binds to the subunit 50S of the bacterial ribosome, and thus inhibits the translocation of peptides. Roxithromycin has similar antimicrobial spectrum as erythromycin, but is more effective against certain gram-negative bacteria, particularly Legionella pneumophila.
Pharmacokinetics
When taken before a meal, roxithromycin is very rapidly absorbed, and diffused into most tissues and phagocytes. Due to the high concentration in phagocytes, roxithromycin is actively transported to the site of infection. During active phagocytosis, large concentrations of roxithromycin are released.
Metabolism
Only a small portion of roxithromycin is metabolised. Most of roxithromycin is secreted unchanged into the bile and some in expired air. Under 10% is excreted into the urine. Roxithromycin's half-life is 12 hours.
Side effects
Most common side effects are gastrointestinal; diarrhoea, nausea, abdominal pain and vomiting. Less common side effects include central or peripheral nervous system events such as headaches, dizziness, vertigo, and also the rarely seen rashes, abnormal liver function values and alteration in senses of smell and taste.
Drug interactions
Roxithromycin has fewer interactions than erythromycin as it has a lower affinity for cytochrome P450.
Roxithromycin does not interact with hormonal contraceptives, prednisolone, carbamazepine, ranitidine or antacids.
When roxithromycin is administered with theophylline, some studies have shown an increase in the plasma concentration of theophylline. A change in dosage is usually not required but patients with high levels of theophylline at the start of the treatment should have their plasma levels monitored.
Roxithromycin appears to interact with warfarin. This is shown by an increase in prothrombin time (international normalised ratio [INR]) in patients taking roxithromycin and warfarin concurrently. As a consequence, severe bleeding episodes have occurred.
Drug Dosage
Adults: 150mg twice in a day, 1-2 hours before meals. For children, it is 2.5 - 5.0mg/kg of body weight, given in two divided doses per day. [2] Mode of Administration- Oral.
References
- ^ "The Effect of 0.5% Roxithromycin Lotion for Androgenetic Alopecia - ClinicalTrials.gov". http://www.clinicaltrials.gov/ct/show/NCT00197379?order=8. Retrieved 2007-09-22.
- ^ Medical pharmacology, Ch.-54, ISBN-81_8448_085_7
Antibacterials: protein synthesis inhibitors (J01A, J01B, J01F, J01G, QJ01XQ) 30S -mycin (Streptomyces)Neomycin# (Framycetin, Paromomycin, Ribostamycin)
Kanamycin# (Amikacin, Arbekacin, Bekanamycin, Dibekacin, Tobramycin)
Paromomycin-micin (Micromonospora)Tetracyclines50S PleuromutilinsErythromycin# • Azithromycin# • Spiramycin • Midecamycin • Oleandomycin • Roxithromycin • Josamycin • Troleandomycin • Clarithromycin • Miocamycin • Rokitamycin • Dirithromycin • Flurithromycin • Ketolide (Telithromycin, Cethromycin, Solithromycin)EF-G Steroid antibacterialsCategories:- Macrolide antibiotics
- InChI=1S/C41H76N2O15/c1-15-29-41(10,49)34(45)24(4)31(42-53-21-52-17-16-50-13)22(2)19-39(8,48)36(58-38-32(44)28(43(11)12)18-23(3)54-38)25(5)33(26(6)37(47)56-29)57-30-20-40(9,51-14)35(46)27(7)55-30/h22-30,32-36,38,44-46,48-49H,15-21H2,1-14H3/b42-31+/t22-,23-,24+,25+,26-,27+,28+,29-,30+,32-,33+,34-,35+,36-,38+,39-,40-,41-/m1/s1
Wikimedia Foundation. 2010.
