- Lymecycline
-
Lymecycline 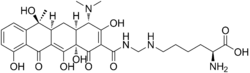
Systematic (IUPAC) name (2S)-6-[[[(Z)-[(4S,4aS,5aS,6S,12aS) -4-(dimethylamino)-6,10,11,12a-tetrahydroxy-6-methyl-1,3,12-trioxo-4,4a,5,5a-tetrahydrotetracen-2-ylidene]-hydroxymethyl]amino]methylamino]-2-aminohexanoic acid Clinical data AHFS/Drugs.com International Drug Names Pregnancy cat. ? Legal status Schedule 4 (Aust) Routes oral Pharmacokinetic data Bioavailability 100% (oral) Metabolism ? Half-life 10 h Excretion renal Identifiers CAS number 992-21-2 
ATC code J01AA04 PubChem CID 24757945 DrugBank APRD00565 ChemSpider 16736147 
UNII 7D6EM3S13P 
KEGG D06884 
Chemical data Formula C29H38N4O10 Mol. mass 602.63 SMILES eMolecules & PubChem  (what is this?) (verify)
(what is this?) (verify)Lymecycline is a tetracycline broad-spectrum antibiotic marketed by the pharmeceutical company Galderma. It is approximately 5000 times more soluble than tetracycline base and is unique amongst tetracyclines in that it is absorbed by an active transport process across the intestinal wall, making use of the same fast and efficient mechanism by which carbohydrates are absorbed.[1]
The greater absorption of lymecycline allows for lower dosages to be used; the standard dose of 408 mg is equivalent to 300 mg tetracycline base, and in its action to 500 mg tetracycline hydrochloride. Lymecycline, unlike tetracycline hydrochloride, is soluble at all physiological pH values.
Contents
History
Lymecycline was released onto the pharmaceutical market in 1963.
Indications
Lymecycline, like other tetracyclines, is used to treat a range of infections. Its better absorption profile makes it preferable to tetracycline for moderately severe acne and typically prescribed for 8 weeks at a time, but alternatives should be sought if no improvement occurs by 3 months.[2]
Dosage
The standard dose is 408 mg (one capsule) daily by mouth. In particularly severe infections this dose may be increased to 1224–1632 mg (three or four capsules) daily.
The 408 mg once daily dosage for acne aids good drug compliance.[clarification needed]
Side effects
Lymecycline's side effects can include rash, headache, diarrhoea, colitis, nausea, vomiting, dermatitis, dysphasia, inflammation of the liver, hypersensitive reactions, and visual disturbances. When taken for a long period of time, it can cause reflux oesophagitis.[3]
References
- ^ New Zealand Datasheet August 2003
- ^ British National Formulary 45 March 2003
- ^ Dr Wang, Peter. "Side effects of Tetralysal". http://www.steadyhealth.com/about/side_effects_of_tetralysal.html. Retrieved 23 March 2011.
See also
Antibacterials: protein synthesis inhibitors (J01A, J01B, J01F, J01G, QJ01XQ) 30S -mycin (Streptomyces)Neomycin# (Framycetin, Paromomycin, Ribostamycin)
Kanamycin# (Amikacin, Arbekacin, Bekanamycin, Dibekacin, Tobramycin)
Paromomycin-micin (Micromonospora)TetracyclinesDoxycycline# •Chlortetracycline • Clomocycline • Demeclocycline • Lymecycline • Meclocycline • Metacycline • Minocycline • Oxytetracycline • Penimepicycline • Rolitetracycline • Tetracycline50S Linezolid • Torezolid • Eperezolid • Posizolid • RadezolidPleuromutilinsRetapamulin • Tiamulin • ValnemulinErythromycin# • Azithromycin# • Spiramycin • Midecamycin • Oleandomycin • Roxithromycin • Josamycin • Troleandomycin • Clarithromycin • Miocamycin • Rokitamycin • Dirithromycin • Flurithromycin • Ketolide (Telithromycin, Cethromycin, Solithromycin)EF-G Steroid antibacterialsCategories:- Tetracycline antibiotics
- Amino acid derivatives
Wikimedia Foundation. 2010.