- Cethromycin
-
Cethromycin 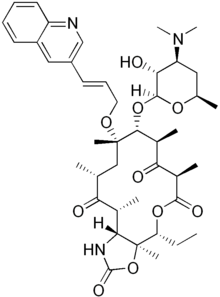
Systematic (IUPAC) name (1S,2R,5R,7R,8R,9S,11R,13R,14R)-8-[(2S,3R,4S,6R)- 4-Dimethylamino-3-hydroxy-6-methyloxan-2-yl]oxy-2-ethyl-1,5,7,9,11,13- hexamethyl-9-[(E)-3-quinolin-3-ylprop-2-enoxy] -3,17-dioxa-15-azabicyclo[12.3.0]heptadecane-4,6,12,16-tetrone Clinical data Pregnancy cat. ? Legal status Phase III Clinical Trials in US Routes Oral Pharmacokinetic data Bioavailability Between 35.8 and 60 % in animal studies. Metabolism Liver Half-life 1.6, 3.0, 4.5, 5.9 and 6 hours. Mouse, Monkey, Rat, Dog and Human respectively. Excretion 7.0% urine 87.2% faeces Identifiers CAS number 205110-48-1 ATC code None PubChem CID 5282045 UNII J0086219X6 
KEGG D02391 
Chemical data Formula C42H59N3O10 Mol. mass 765.931 g/mol Physical data Melt. point 211–213 °C (412–415 °F)  (what is this?) (verify)
(what is this?) (verify)Cethromycin (initially known as ABT-773[1][2]) is a ketolide antibiotic undergoing research for the treatment of community acquired pneumonia (CAP)[1][3][4][5] and for the prevention of post-exposure inhalational anthrax, and was given an "orphan drug" status for this indication.[6] Originally discovered and developed by Abbott, it was acquired by Advanced Life Sciences Inc. for further development.
On October 1, 2008 Advanced Life Sciences submitted a New Drug Application (NDA) to Food and Drug Administration (FDA) for cethromycin to treat mild-to-moderate community acquired pneumonia.[7]
On December 3, 2008 Advanced Life Sciences announced that this New Drug Application has been accepted for filing by the FDA.[8]
References
- ^ a b Lawrence LE (June 2001). "ABT-773 (Abbott Laboratories)". Current Opinion in Investigational Drugs 2 (6): 766–72. PMID 11572654.
- ^ Dougherty TJ, Barrett JF (February 2001). "ABT-773: a new ketolide antibiotic". Expert Opinion on Investigational Drugs 10 (2): 343–51. doi:10.1517/13543784.10.2.343. PMID 11178346.
- ^ Zhanel GG, Hisanaga T, Nichol K, Wierzbowski A, Hoban DJ (November 2003). "Ketolides: an emerging treatment for macrolide-resistant respiratory infections, focusing on S. pneumoniae". Expert Opinion on Emerging Drugs 8 (2): 297–321. doi:10.1517/14728214.8.2.297. PMID 14661991.
- ^ Reinert RR (June 2004). "Clinical efficacy of ketolides in the treatment of respiratory tract infections". The Journal of Antimicrobial Chemotherapy 53 (6): 918–27. doi:10.1093/jac/dkh169. PMID 15117934.
- ^ Hammerschlag MR, Sharma R (March 2008). "Use of cethromycin, a new ketolide, for treatment of community-acquired respiratory infections". Expert Opinion on Investigational Drugs 17 (3): 387–400. doi:10.1517/13543784.17.3.387. PMID 18321237.
- ^ Cethromycin - Advanced Life Sciences
- ^ Cethromycin New Drug Application
- ^ Cethromycin New Drug Application accepted for filing by FDA
Further reading
Antibacterials: protein synthesis inhibitors (J01A, J01B, J01F, J01G, QJ01XQ) 30S -mycin (Streptomyces)Neomycin# (Framycetin, Paromomycin, Ribostamycin)
Kanamycin# (Amikacin, Arbekacin, Bekanamycin, Dibekacin, Tobramycin)
Paromomycin-micin (Micromonospora)Tetracyclines50S PleuromutilinsErythromycin# • Azithromycin# • Spiramycin • Midecamycin • Oleandomycin • Roxithromycin • Josamycin • Troleandomycin • Clarithromycin • Miocamycin • Rokitamycin • Dirithromycin • Flurithromycin • Ketolide (Telithromycin, Cethromycin, Solithromycin)EF-G Steroid antibacterials#WHO-EM. ‡Withdrawn from market. Clinical trials: †Phase III. §Never to phase III 
This systemic antibacterial-related article is a stub. You can help Wikipedia by expanding it.
