- 2-Oxazolidone
-
2-Oxazolidone 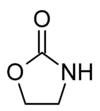
 1,3-Oxazolidin-2-oneOther names2-Oxazolidone
1,3-Oxazolidin-2-oneOther names2-Oxazolidone
2-OxazolidinoneIdentifiers CAS number 497-25-6 
PubChem 73949 ChemSpider 66579 
KEGG C06695 
Jmol-3D images Image 1 - O=C1OCCN1
Properties Molecular formula C3H5NO2 Molar mass 87.077 g/mol Appearance Solid Melting point 86–89 °C
Boiling point 220 °C at 48 torr
Related compounds Related compounds Oxazolidine  (verify) (what is:
(verify) (what is:  /
/ ?)
?)
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references 2-Oxazolidone is a heterocyclic organic compound containing both nitrogen and oxygen in a 5-membered ring.
Contents
Oxazolidinones
Evans auxiliaries
Oxazolidinones are a class of compounds containing 2-oxazolidone in the structure. In chemistry, they are useful as Evans auxiliaries, which are used for chiral synthesis. Usually, the acid chloride substrate reacts with the oxazolidinone to form an imide. Substituents at the 4 and 5 position of the oxazolidinone direct any aldol reaction to the alpha position of the carbonyl of the substrate.
Pharmaceuticals
Oxazolidinones are mainly used as antibiotics. The antibacterial effect of oxazolidinones is by working as protein synthesis inhibitors, targeting an early step involving the binding of N-formylmethionyl-tRNA to the ribosome.[1] Some of the most important oxazolidinones are the last generation of antibiotics used against gram-positive pathogens, including superbugs such as Methicillin-resistant Staphylococcus aureus. These antibiotics are considered as a choice of last resort where every other antibiotic therapy has failed.
Examples of antibiotic oxazolidinones include:
- Linezolid (Zyvox), which is available for intravenous administration and also has the advantage of having excellent oral bioavailability.
- Posizolid, which appears to have excellent, targeted bactericidal activity against all common gram-positive bacteria, regardless of resistance to other classes of antibiotics.
- Torezolid is in phase-II clinical trials
- Radezolid (RX-1741) has completed some phase-II clinical trials.[2]
- Cycloserine is a second line drug against tuberculosis
An oxazolidinone derivative used for other purposes is rivaroxaban, which is approvedby the FDA for venous thromboembolism prophylaxis.
History
The first ever used oxazolidinone was Cycloserine (4-amino-1,2-oxazolidin-3-one), a second line drug against Tuberculosis since 1956.[3] Developed during the nineties when several bacterial strains were becoming resistant against such antibiotics as vancomycin. Linezolid (Zyvox) is the first approved agent in the class.
The first commercially available 1,3-oxazolidinone antibiotic was Linezolid, discovered and developed by Pharmacia & Upjohn.
In 2002 AstraZeneca introduced posizolid (AZD2563).
References
- ^ Shinabarger, D. (1999). "Mechanism of action of the oxazolidinone antibacterial agents". Expert Opinion on Investigational Drugs 8 (8): 1195–1202. doi:10.1517/13543784.8.8.1195. PMID 15992144.
- ^ "Rx 1741". Rib-X Pharmaceuticals. 2009. http://www.rib-x.com/pipeline/rx_1741. Retrieved 2009-05-17.
- ^ A. W. Frahm, H. H. J. Hager, F. v. Bruchhausen, M. Albinus, H. Hager: Hagers Handbuch der pharmazeutischen Praxis: Folgeband 4: Stoffe A-K., Birkhäuser, 1999, ISBN 978-3-540-52688-9
External links
Antibacterials: protein synthesis inhibitors (J01A, J01B, J01F, J01G, QJ01XQ) 30S -mycin (Streptomyces)Neomycin# (Framycetin, Paromomycin, Ribostamycin)
Kanamycin# (Amikacin, Arbekacin, Bekanamycin, Dibekacin, Tobramycin)
Paromomycin-micin (Micromonospora)Tetracyclines50S Oxazolidinone
(initiation inhibitors)Linezolid • Torezolid • Eperezolid • Posizolid • RadezolidPleuromutilinsRetapamulin • Tiamulin • ValnemulinErythromycin# • Azithromycin# • Spiramycin • Midecamycin • Oleandomycin • Roxithromycin • Josamycin • Troleandomycin • Clarithromycin • Miocamycin • Rokitamycin • Dirithromycin • Flurithromycin • Ketolide (Telithromycin, Cethromycin, Solithromycin)EF-G Steroid antibacterialsCategories:- Articles with validated CAS Registry Numbers
- Oxazolidinone antibiotics
Wikimedia Foundation. 2010.