- Ethambutol
-
Ethambutol 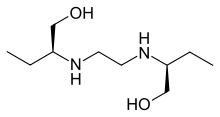
Systematic (IUPAC) name (2S,2′S)-2,2′-(ethane-1,2-diyldiimino)dibutan-1-ol Clinical data Trade names Myambutol AHFS/Drugs.com monograph MedlinePlus a682550 Pregnancy cat. B Legal status ? Routes Oral Pharmacokinetic data Bioavailability Well-absorbed from GI tract Protein binding 20 to 30% Metabolism Liver Half-life 3-4 hr (increased in impaired renal function) Excretion Renal Identifiers CAS number 74-55-5 
ATC code J04AK02 PubChem CID 14052 DrugBank APRD00957 ChemSpider 13433 
UNII 8G167061QZ 
KEGG D07925 
ChEBI CHEBI:4877 
ChEMBL CHEMBL44884 
Chemical data Formula C10H24N2O2 Mol. mass 204.31 g/mol SMILES eMolecules & PubChem  (what is this?) (verify)
(what is this?) (verify)Ethambutol (commonly abbreviated EMB or simply E) is a bacteriostatic antimycobacterial drug prescribed to treat tuberculosis.[1] It is usually given in combination with other tuberculosis drugs, such as isoniazid, rifampicin and pyrazinamide.
It is sold under the trade names Myambutol and Servambutol.
Contents
Adverse effects
- Optic neuritis.[2] Hence contraindicated in children below 6 yrs of age.
- Red-green color blindness
- Peripheral neuropathy
- Arthralgia
- Hyperuricaemia
- vertical nystagmus
- milk skin reaction
Mechanism of action
Ethambutol is bacteriostatic against actively growing TB bacilli. It works by obstructing the formation of cell wall. Mycolic acids attach to the 5'-hydroxyl groups of D-arabinose residues of arabinogalactan and form mycolyl-arabinogalactan-peptidoglycan complex in the cell wall. It disrupts arabinogalactan synthesis by inhibiting the enzyme arabinosyl transferase. Disruption of the arabinogalactan synthesis inhibits the formation of this complex and leads to increased permeability of the cell wall.
Pharmacokinetics
It is well absorbed from the gastrointestinal tract and well distributed in body tissues and fluids, 50% is excreted unchanged in urine..
References
- ^ Yendapally R, Lee RE (March 2008). "Design, synthesis, and evaluation of novel ethambutol analogues". Bioorg. Med. Chem. Lett. 18 (5): 1607–11. doi:10.1016/j.bmcl.2008.01.065. PMC 2276401. PMID 18242089. http://linkinghub.elsevier.com/retrieve/pii/S0960-894X(08)00082-6.
- ^ Lim SA (April 2006). "Ethambutol-associated optic neuropathy". Ann. Acad. Med. Singap. 35 (4): 274–8. PMID 16710500. http://www.annals.edu.sg/pdf/35VolNo4200605/V35N4p274.pdf.
External links
Antimycobacterials, including tuberculosis treatment and leprostatic agents (J04) Nucleic acid inhibitor Antifolates/DSIASAProtein synthesis inhibitor Cell envelope antibiotic Peptidoglycan layerArabinogalactan layerEthylenediamine/arabinosyltransferase inhibitor: Ethambutol#
SQ109†Mycolic acid layerHydrazides/mycolic acid synth. inhibition: Isoniazid#
Thiocarbamides: Ethionamide# • Prothionamide • ThiocarlideOther/unknown phenazine (Clofazimine)# • pyrazine (Pyrazinamide#, Morinamide) • isoxazole (Terizidone) • R207910/TMC207†Combinations Rifampicin/isoniazid/pyrazinamideCategories:- Antibiotics
- Tuberculosis
- World Health Organization essential medicines
- Alcohols
Wikimedia Foundation. 2010.
