- Magnesium stearate
-
Magnesium stearate 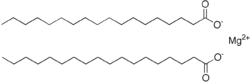 Magnesium octadecanoate
Magnesium octadecanoateIdentifiers CAS number 557-04-0 
PubChem 11177 ChemSpider 10704 
UNII 70097M6I30 
ChEBI CHEBI:9254 
Jmol-3D images Image 1 - [Mg+2].[O-]C(=O)CCCCCCCCCCCCCCCCC.[O-]C(=O)CCCCCCCCCCCCCCCCC
Properties Molecular formula Mg(C18H35O2)2 Molar mass 591.27 g/mol Appearance light white powder Odor slight Melting point 120 °C, 393 K, 248 °F
Solubility in water negligible Solubility insoluble in ether
slightly soluble in benzeneHazards MSDS External MSDS  stearate (verify) (what is:
stearate (verify) (what is:  /
/ ?)
?)
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references Magnesium stearate, also called octadecanoic acid, magnesium salt, is a white substance which is solid at room temperature. It has the chemical formula Mg(C18H35O2)2. It is a salt containing two equivalents of stearate (the anion of stearic acid) and one magnesium cation (Mg2+). Magnesium stearate melts at about 120 °C, is not soluble in water, and is generally considered safe for human consumption at levels below 2500 mg/kg per day.[1] In 1979, FDA's Subcommittee on GRAS (generally recognized as safe) Substances (SCOGS) reported, "There is no evidence in the available information on ... magnesium stearate ... that demonstrates, or suggests reasonable grounds to suspect, a hazard to the public when they are used at levels that are now current and in the manner now practiced, or which might reasonably be expected in the future."[2]
Magnesium stearate is often used as a diluent[3] in the manufacture of medical tablets, capsules and powders.[4] In this regard, the substance is also useful, because it has lubricating properties, preventing ingredients from sticking to manufacturing equipment during the compression of chemical powders into solid tablets; magnesium stearate is the most commonly used lubricant for tablets.[5] Studies have shown that magnesium stearate may affect the release time of the active ingredients in tablets, etc., but not that it reduces the over-all bioavailability of those ingredients.[6][7] As a food additive or pharmaceutical excipient, its E number is E470b.
Magnesium stearate is also used to bind sugar in hard candies and is a common ingredient in baby formulas. In pure powder form, the substance can be a dust explosion hazard, although this issue is effectively insignificant beyond the manufacturing plants using it.[8]
Magnesium stearate is manufactured from both animal and vegetable oils. Some nutritional supplements specify that the magnesium stearate used is sourced from vegetables.
Magnesium stearate is a major component of "bathtub rings". When produced by soap and hard water, magnesium stearate and calcium stearate both form a white solid insoluble in water, and are collectively known as "soap scum".[9]
References
- ^ D. Søndergaarda, O. Meyera and G. Würtzena (1980). "Magnesium stearate given peroprally to rats. A short term study". Toxicology 17 (1): 51–55. doi:10.1016/0300-483X(80)90026-8. PMID 7434368.
- ^ FDA's SCOGS Database; Report No. 60; ID Code: 557-04-0; Year: 1979
- ^ Steve Ritter (2008). "What's That Stuff? Excipients: Inactive ingredients in medicines serve multiple functions in drug delivery". Chemical & Engineering News 86 (1): 25. doi:10.1021/cen-v086n001.p025. http://pubs.acs.org/cen/whatstuff/86/8601sci3.html.
- ^ Sworbrick, James; James C. Boylan (1990). Encyclopedia of pharmaceutical technology. p. 2274. ISBN 0824728246, 9780824728243.
- ^ Weiner, Myra L.; Lois A. Kotkoskie (1999). Excipient Toxicity and Safety. p. 10. ISBN 0824782100, 9780824782108.
- ^ Alija Uzunović, Edina Vranić; "Effect Of Magnesium Stearate Concentration On Dissolution Properties Of Ranitidine Hydrochloride Coated Tablets"; Bosnian Journal Of Basic Medical Sciences, 2007, 7(3): 279-283
- ^ Natalie D. Eddington, Muhammad Ashraf, Larry L. Augsburger, James L. Leslie, Michael J. Fossler, Lawrence J. Lesko, Vinod P. Shah, Gurvinder Singh Rekhi; "Identification of Formulation and Manufacturing Variables That Influence In Vitro Dissolution and In Vivo Bioavailability of Propranolol Hydrochloride Tablets"; Pharmaceutical Development and Technology, Volume 3, Issue 4 November 1998 , pages 535 - 547
- ^ International Chemical Safety Card 1403
- ^ Anne Marie Helmenstine; About.com: "Why Is It Harder to Rinse off Soap with Soft Water?"; retrieved 19 Mar 2010
Categories:- Excipients
- Magnesium compounds
- Stearates
Wikimedia Foundation. 2010.
