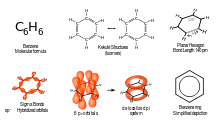- Benzene
-
For other uses, see Benzene (disambiguation).See also: Benzole
Benzene 
 BenzeneOther namesBenzol
BenzeneOther namesBenzol
cyclohexa-1,3,5-trieneIdentifiers CAS number 71-43-2 
PubChem 241 ChemSpider 236 
UNII J64922108F 
KEGG C01407 
ChEBI CHEBI:16716 
ChEMBL CHEMBL277500 
RTECS number CY1400000 Jmol-3D images Image 1 - c1ccccc1
Properties Molecular formula C6H6 Molar mass 78.11 g mol−1 Appearance Colorless liquid Density 0.8765(20) g/cm3 [1] Melting point 5.5 °C, 278.7 K
Boiling point 80.1 °C, 353.3 K
Solubility in water 1.8 g/L (15 °C) [2][3][4] λmax 255 nm Viscosity 0.652 cP at 20 °C Dipole moment 0 D Hazards MSDS External MSDS EU classification Flammable (F)
Carc. Cat. 1
Muta. Cat. 2
Toxic (T)R-phrases R45, R46, R11, R36/38,R48/23/24/25, R65 S-phrases S53, S45 NFPA 704 Flash point −11 °C, 262 K Related compounds Related compounds toluene
borazineSupplementary data page Structure and
propertiesn, εr, etc. Thermodynamic
dataPhase behaviour
Solid, liquid, gasSpectral data UV, IR, NMR, MS  (verify) (what is:
(verify) (what is:  /
/ ?)
?)
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references Benzene is an organic chemical compound. It is composed of 6 carbon atoms in a ring, with 1 hydrogen atom attached to each carbon atom, with the molecular formula C6H6.
Benzene is a natural constituent of crude oil, and is one of the most basic petrochemicals. Benzene is an aromatic hydrocarbon and the second [n]-annulene ([6]-annulene), a cyclic hydrocarbon with a continuous pi bond. It is sometimes abbreviated Ph–H. Benzene is a colorless and highly flammable liquid with a sweet smell. Because it is a known carcinogen, its use as an additive in gasoline is now limited, but it is an important industrial solvent and precursor to basic industrial chemicals including drugs, plastics, synthetic rubber, and dyes.
Contents
History
Discovery
The word "benzene" derives historically from "gum benzoin", sometimes called "benjamin" (i.e., benzoin resin), an aromatic resin known to European pharmacists and perfumers since the 15th century as a product of southeast Asia. "Benzoin" is itself a corruption of the Arabic expression "luban jawi", or "frankincense of Java". An acidic material was derived from benzoin by sublimation, and named "flowers of benzoin", or benzoic acid. The hydrocarbon derived from benzoic acid thus acquired the name benzin, benzol, or benzene.[5]
Michael Faraday first isolated and identified benzene in 1825 from the oily residue derived from the production of illuminating gas, giving it the name bicarburet of hydrogen.[6][7]
In 1833, Eilhard Mitscherlich produced it via the distillation of benzoic acid (from gum benzoin) and lime. He gave the compound the name benzin.[8]
In 1836, the French chemist Auguste Laurent named the substance "phène"; this is the root of the word phenol, which is hydroxylated benzene, and phenyl, which is the radical formed by abstraction of a hydrogen atom (free radical H*) from benzene.
 Historic benzene formulae as proposed by Kekulé.[9]
Historic benzene formulae as proposed by Kekulé.[9]
In 1845, Charles Mansfield, working under August Wilhelm von Hofmann, isolated benzene from coal tar. Four years later, Mansfield began the first industrial-scale production of benzene, based on the coal-tar method. Gradually the sense developed among chemists that substances related to benzene represent a diverse chemical family. In 1855 August Wilhelm Hofmann used the word "aromatic" to designate this family relationship, after a characteristic property of many of its members.
Ring formula
The empirical formula for benzene was long known, but its highly polyunsaturated structure, with just one hydrogen atom for each carbon atom, was challenging to determine. Archibald Scott Couper in 1858 and Joseph Loschmidt in 1861 suggested possible structures that contained multiple double bonds or multiple rings, but too little evidence was then available to help chemists decide on any particular structure.
In 1865, the German chemist Friedrich August Kekulé published a paper in French (for he was then teaching in Francophone Belgium) suggesting that the structure contained a six-membered ring of carbon atoms with alternating single and double bonds. The next year he published a much longer paper in German on the same subject.[10][11] Kekulé used evidence that had accumulated in the intervening years—namely, that there always appeared to be only one isomer of any monoderivative of benzene, and that there always appeared to be exactly three isomers of every diderivative—now understood to correspond to the ortho, meta, and para patterns of arene substitution—to argue in support of his proposed structure. Kekulé's symmetrical ring could explain these curious facts, as well as benzene's 1:1 carbon-hydrogen ratio.
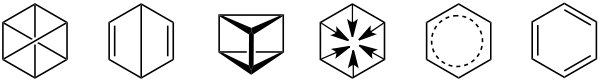 Historic benzene formulae (from left to right) by Claus (1867), Dewar (1867), Ladenburg (1869), Armstrong (1887), Thiele (1899) and Kekulé (1865). Dewar benzene and prismane are different chemicals that have Dewar's and Ladenburg's structures. Thiele and Kekulé's structures are used today.
Historic benzene formulae (from left to right) by Claus (1867), Dewar (1867), Ladenburg (1869), Armstrong (1887), Thiele (1899) and Kekulé (1865). Dewar benzene and prismane are different chemicals that have Dewar's and Ladenburg's structures. Thiele and Kekulé's structures are used today.
The new understanding of benzene, and hence of all aromatic compounds, proved to be so important for both pure and applied chemistry that in 1890 the German Chemical Society organized an elaborate appreciation in Kekulé's honor, celebrating the twenty-fifth anniversary of his first benzene paper. Here Kekulé spoke of the creation of the theory. He said that he had discovered the ring shape of the benzene molecule after having a reverie or day-dream of a snake seizing its own tail (this is a common symbol in many ancient cultures known as the Ouroboros or Endless knot). This vision, he said, came to him after years of studying the nature of carbon-carbon bonds. This was 7 years after he had solved the problem of how carbon atoms could bond to up to four other atoms at the same time. It is curious that a similar, humorous depiction of benzene had appeared in 1886 in the Berichte der Durstigen Chemischen Gesellschaft (Journal of the Thirsty Chemical Society), a parody of the Berichte der Deutschen Chemischen Gesellschaft, only the parody had monkeys seizing each other in a circle, rather than snakes as in Kekulé's anecdote.[12] Some historians have suggested that the parody was a lampoon of the snake anecdote, possibly already well-known through oral transmission even if it had not yet appeared in print.[5] Others have speculated that Kekulé's story in 1890 was a re-parody of the monkey spoof, and was a mere invention rather than a recollection of an event in his life. Kekulé's 1890 speech[13] in which these anecdotes appeared has been translated into English.[14] If one takes the anecdote as the memory of a real event, circumstances mentioned in the story suggest that it must have happened early in 1862.[15]
The cyclic nature of benzene was finally confirmed by the crystallographer Kathleen Lonsdale in 1929.[16][17]
Structure
Main article: AromaticityBenzene represents a special problem in that, to account for all the bonds, there must be alternating double carbon bonds:[18]
X-ray diffraction shows that all of six carbon-carbon bonds in benzene are of the same length of 140 picometres (pm). The C–C bond lengths are greater than a double bond (135 pm) but shorter than a single bond (147 pm). This intermediate distance is consistent with electron delocalization: the electrons for C–C bonding are distributed equally between each of the six carbon atoms. The molecule is planar.[19] One representation is that the structure exists as a superposition of so-called resonance structures, rather than either form individually. The delocalization of electrons is one explanation for the thermodynamic stability of benzene and related aromatic compounds. It is likely that this stability contributes to the peculiar molecular and chemical properties known as aromaticity. To indicate the delocalized nature of the bonding, benzene is often depicted with a circle inside a hexagonal arrangement of carbon atoms:The delocalized picture of benzene has been contested by Cooper, Gerratt and Raimondi in their article published in 1986 in the journal Nature. They showed that the electrons in benzene are almost certainly localized, and the aromatic properties of benzene originate from spin coupling rather than electron delocalization.[20] This view has been supported in the next-year Nature issue,[21][22][23] but it has been slow to permeate the general chemistry community.
As is common in organic chemistry, the carbon atoms in the diagram above have been left unlabeled. Realizing each carbon has 2p electrons, each carbon donates an electron into the delocalized ring above and below the benzene ring. It is the side-on overlap of p-orbitals that produces the pi clouds.
Derivatives of benzene occur sufficiently often as a component of organic molecules that there is a Unicode symbol in the Miscellaneous Technical block with the code U+232C (⌬) to represent it with three double bonds,[24] and U+23E3 (⏣) for a delocalized version.[25]
Substituted benzene derivatives
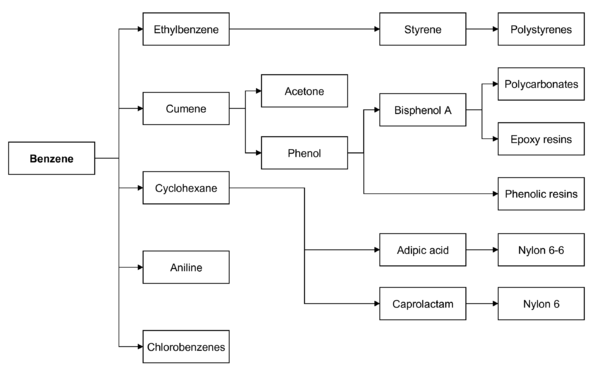
Main article: Aromatic hydrocarbonsMany important chemical compounds are derived from benzene by replacing one or more of its hydrogen atoms with another functional group. Examples of simple benzene derivatives are phenol, toluene, and aniline, abbreviated PhOH, PhMe, and PhNH2, respectively. Linking benzene rings gives biphenyl, C6H5–C6H5. Further loss of hydrogen gives "fused" aromatic hydrocarbons, such as naphthalene and anthracene. The limit of the fusion process is the hydrogen-free allotrope of carbon, graphite.
In heterocycles, carbon atoms in the benzene ring are replaced with other elements. The most important derivatives are the rings containing nitrogen. Replacing one CH with N gives the compound pyridine, C5H5N. Although benzene and pyridine are structurally related, benzene cannot be converted into pyridine. Replacement of a second CH bond with N gives, depending on the location of the second N, pyridazine, pyrimidine, and pyrazine.
Production
Four chemical processes contribute to industrial benzene production: catalytic reforming, toluene hydrodealkylation, toluene disproportionation, and steam cracking. According to the ATSDR Toxicological Profile for benzene, between 1978 and 1981, catalytic reformats accounted for approximately 44–50% of the total U.S benzene production.
Until World War II, most benzene was produced as a by-product of coke production (or "coke-oven light oil") in the steel industry. However, in the 1950s, increased demand for benzene, especially from the growing polymers industry, necessitated the production of benzene from petroleum. Today, most benzene comes from the petrochemical industry, with only a small fraction being produced from coal.
Catalytic reforming
In catalytic reforming, a mixture of hydrocarbons with boiling points between 60–200 °C is blended with hydrogen gas and then exposed to a bifunctional platinum chloride or rhenium chloride catalyst at 500–525 °C and pressures ranging from 8–50 atm. Under these conditions, aliphatic hydrocarbons form rings and lose hydrogen to become aromatic hydrocarbons. The aromatic products of the reaction are then separated from the reaction mixture (or reformate) by extraction with any one of a number of solvents, including diethylene glycol or sulfolane, and benzene is then separated from the other aromatics by distillation. The extraction step of aromatics from the reformate is designed to produce aromatics with lowest non-aromatic components. So-called BTX (benzene-toluene-xylene) process consists of such extraction and distillation steps. One such widely used process from UOP was licensed to producers and called the Udex process.
In similar fashion to this catalytic reforming, UOP and BP commercialized a method from LPG (mainly propane and butane) to aromatics.
Toluene hydrodealkylation
Toluene hydrodealkylation converts toluene to benzene. In this hydrogen-intensive process, toluene is mixed with hydrogen, then passed over a chromium, molybdenum, or platinum oxide catalyst at 500–600 °C and 40–60 atm pressure. Sometimes, higher temperatures are used instead of a catalyst (at the similar reaction condition). Under these conditions, toluene undergoes dealkylation to benzene and methane:
- C6H5CH3 + H2 → C6H6 + CH4
This irreversible reaction is accompanied by an equilibrium side reaction that produces biphenyl (aka diphenyl) at higher temperature:
If the raw material stream contains much non-aromatic components (paraffins or naphthenes), those are likely decomposed to lower hydrocarbons such as methane, which increases the consumption of hydrogen.
A typical reaction yield exceeds 95%. Sometimes, xylenes and heavier aromatics are used in place of toluene, with similar efficiency.
This is often called "on-purpose" methodology to produce benzene, compared to conventional BTX (benzene-toluene-xylene) processes.
Toluene disproportionation
Where a chemical complex has similar demands for both benzene and xylene, then toluene disproportionation (TDP) may be an attractive alternative to the toluene hydrodealkylation. In the broad sense, 2 toluene molecules are reacted and the methyl groups rearranged from one toluene molecule to the other, yielding one benzene molecule and one xylene molecule.
Given that demand for para-xylene (p-xylene) substantially exceeds demand for other xylene isomers, a refinement of the TDP process called Selective TDP (STDP) may be used. In this process, the xylene stream exiting the TDP unit is approximately 90% paraxylene. In some current catalytic systems, even the benzene-to-xylenes ratio is decreased (more xylenes) when the demand of xylenes is higher.
Steam cracking
Steam cracking is the process for producing ethylene and other alkenes from aliphatic hydrocarbons. Depending on the feedstock used to produce the olefins, steam cracking can produce a benzene-rich liquid by-product called pyrolysis gasoline. Pyrolysis gasoline can be blended with other hydrocarbons as a gasoline additive, or distilled (in BTX process) to separate it into its components, including benzene.
Other sources
Trace amounts of benzene may result whenever carbon-rich materials undergo incomplete combustion. It is produced in volcanoes and forest fires, and is also a component of cigarette smoke. Benzene is a principal product from the combustion of PVC (polyvinyl chloride).
Uses
Early uses
In the 19th and early-20th centuries, benzene was used as an after-shave lotion because of its pleasant smell. Prior to the 1920s, benzene was frequently used as an industrial solvent, especially for degreasing metal. As its toxicity became obvious, benzene was supplanted by other solvents, especially toluene (methyl benzene), which has similar physical properties but is not as carcinogenic.
In 1903, Ludwig Roselius popularized the use of benzene to decaffeinate coffee. This discovery led to the production of Sanka (the letters "ka" in the brand name stand for kaffein). This process was later discontinued. Benzene was historically used as a significant component in many consumer products such as Liquid Wrench, several paint strippers, rubber cements, spot removers and other hydrocarbon-containing products. Some ceased manufacture of their benzene-containing formulations in about 1950, while others continued to use benzene as a component or significant contaminant until the late 1970s when leukemia deaths were found associated with Goodyear's Pliofilm production operations in Ohio. Until the late 1970s, many hardware stores, paint stores, and other retail outlets sold benzene in small cans, such as quart size, for general-purpose use. Many students were exposed to benzene in school and university courses while performing laboratory experiments with little or no ventilation in many cases. This very dangerous practice has been almost totally eliminated.
As a gasoline (petrol) additive, benzene increases the octane rating and reduces knocking. As a consequence, gasoline often contained several percent benzene before the 1950s, when tetraethyl lead replaced it as the most widely-used antiknock additive. With the global phaseout of leaded gasoline, benzene has made a comeback as a gasoline additive in some nations. In the United States, concern over its negative health effects and the possibility of benzene's entering the groundwater have led to stringent regulation of gasoline's benzene content, with limits typically around 1%.[26] European petrol specifications now contain the same 1% limit on benzene content. The United States Environmental Protection Agency has new regulations that will lower the benzene content in gasoline to 0.62% in 2011.[27]
Current uses
Today, benzene is used mainly as an intermediate to make other chemicals. Its most widely-produced derivatives include styrene, which is used to make polymers and plastics, phenol for resins and adhesives (via cumene), and cyclohexane, which is used in the manufacture of Nylon. Smaller amounts of benzene are used to make some types of rubbers, lubricants, dyes, detergents, drugs, explosives, napalm, and pesticides.
In both the US and Europe, 50% of benzene is used in the production of ethylbenzene/styrene, 20% is used in the production of cumene, and about 15% of benzene is used in the production of cyclohexane (eventually to nylon).[citation needed]
In laboratory research, toluene is now often used as a substitute for benzene. The solvent-properties of the two are similar, but toluene is less toxic and has a wider liquid range.
Benzene has been used as a basic research tool in a variety of experiments including analysis of a two-dimensional gas.
Reactions
The most common reactions that benzene undergoes are substitution reactions.[28]
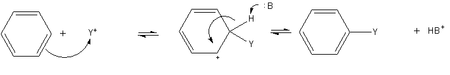
- Electrophilic aromatic substitution is a general method of derivatizing benzene. Benzene is sufficiently nucleophilic that it undergoes substitution by acylium ions or alkyl carbocations to give substituted derivatives.
-
- The Friedel-Crafts alkylation involves the alkylation of benzene (and many other aromatic rings) using an alkyl halide in the presence of a strong Lewis acid catalyst.
-
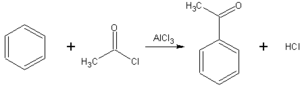
- The Friedel-Crafts acylation is a specific example of electrophilic aromatic substitution. The reaction involves the acylation of benzene (or many other aromatic rings) with an acyl chloride using a strong Lewis acid catalyst such as aluminium chloride or Iron(III) chloride, which act as a halogen carrier.
-
- Sulfonation. The most common method involves mixing sulfuric acid with Sulfur trioxide, a mixture called fuming sulfuric acid. The sulfuric acid protonates either itself or Sulfur trioxide, giving the sulfur atom a permanent, rather than resonance stabilized positive formal charge. This molecule is electrophilic enough to be attacked by the aromatic ring, the electrophilic aromatic substitution occurs.
- Nitration: Benzene undergoes nitration with nitronium ions (NO2+) as the electrophile. Thus, warming benzene at 50–55 °C, with a combination of concentrated sulfuric and nitric acid to produce the electrophile, gives nitrobenzene.
- Hydrogenation (reduction): Benzene and derivatives convert to cyclohexane and derivatives when treated with hydrogen at 450 K and 10 atm of pressure with a finely divided nickel catalyst.
- Benzene is an excellent ligand in the organometallic chemistry of low-valent metals. Important examples include the sandwich and half-sandwich complexes, respectively, Cr(C6H6)2 and [RuCl2(C6H6)]2.
Environmental transformation
Even if it is not a common substrate for the metabolism of organisms, benzene could be oxidized by both bacteria and eukaryotes.
In bacteria, dioxygenase enzyme can add an oxygen molecule to the ring, and the unstable product is immediately reduced (by NADH) to a cyclic diol with two double bonds, breaking the aromaticity. Next, the diol is newly reduced by NADH to catechol.
The catechol is then metabolized to acetyl CoA and succinyl CoA, used by organisms mainly in the Krebs Cycle for energy production.
Health effects
Benzene causes cancer and other illnesses. Benzene is a "notorious cause" of bone marrow failure. "Vast quantities of epidemiologic, clinical, and laboratory data" link benzene to aplastic anemia, acute leukemia, and bone marrow abnormalities.[29][30]
The American Petroleum Institute (API) stated in 1948 that "it is generally considered that the only absolutely safe concentration for benzene is zero."[31] The US Department of Health and Human Services (DHHS) classifies benzene as a human carcinogen. Long-term exposure to excessive levels of benzene in the air causes leukemia, a potentially fatal cancer of the blood-forming organs, in susceptible individuals. In particular, Acute myeloid leukemia or acute non-lymphocytic leukaemia (AML & ANLL) is not disputed to be caused by benzene.[32] IARC rated benzene as "known to be carcinogenic to humans" (Group 1).
Outdoor air may contain low levels of benzene from automobile service stations, wood smoke, tobacco smoke, the transfer of gasoline, exhaust from motor vehicles, and industrial emissions.[33] About 50% of the entire nationwide (United States) exposure to benzene results from smoking tobacco or from exposure to tobacco smoke.[34]
Vapors from products that contain benzene, such as glues, paints, furniture wax, and detergents, can also be a source of exposure, although many of these have been modified or reformulated since the late 1970s to eliminate or reduce the benzene content. Air around hazardous waste sites or gas stations may contain higher levels of benzene. Because petroleum hydrocarbon products are complex mixtures of chemicals, risk assessments for these products, in general, focus on specific toxic constituents. The petroleum constituents of primary interest to human health have been the aromatic hydrocarbons (i.e., benzene, ethylbenzene, toluene, and xylenes). OSHA requires that a mixture "shall be assumed to present a carcinogenic hazard if it contains a component in concentrations of 0.1% or greater, which is considered to be a carcinogen.[35][36]
The short-term breathing of high levels of benzene can result in death; low levels can cause drowsiness, dizziness, rapid heart rate, headaches, tremors, confusion, and unconsciousness. Eating or drinking foods containing high levels of benzene can cause vomiting, irritation of the stomach, dizziness, sleepiness, convulsions, and death.
The major effects of benzene are manifested via chronic (long-term) exposure through the blood. Benzene damages the bone marrow and can cause a decrease in red blood cells, leading to anemia. It can also cause excessive bleeding and depress the immune system, increasing the chance of infection. Benzene causes leukemia and is associated with other blood cancers and pre-cancers of the blood.
Human exposure to benzene is a global health problem. Benzene targets liver, kidney, lung, heart and the brain and can cause DNA strand breaks, chromosomal damage, etc. Benzene causes cancer in both animals and humans. Benzene was first reported to induce cancer in humans in the 1920s. The chemical industry claims it was not until 1979 that the cancer-inducing properties were determined "conclusively" in humans, despite many references to this fact in the medical literature. Industry exploited this "discrepancy" and tried to discredit animal studies that showed that benzene causes cancer, saying that they are not relevant to humans. Benzene has been shown to cause cancer in both sexes of multiple species of laboratory animals exposed via various routes.[37][38]
Some women having breathed high levels of benzene for many months had irregular menstrual periods and a decrease in the size of their ovaries. Benzene exposure has been linked directly to the neural birth defects spina bifida and anencephaly.[39] Men exposed to high levels of benzene are more likely to have an abnormal amount of chromosomes in their sperm, which impacts fertility and fetal development.[40]
Animal studies have shown low birth weights, delayed bone formation, and bone marrow damage when pregnant animals breathed benzene.
Benzene has been connected to a rare form of kidney cancer in two separate studies, one involving tank truck drivers, and the other involving seamen on tanker vessels, both carrying benzene-laden chemicals.
Exposure to benzene
Workers in various industries that make or use benzene may be at risk for being exposed to high levels of this carcinogenic chemical. Industries that involve the use of benzene include the rubber industry, oil refineries, coke and chemical plants, shoe manufacturers, and gasoline-related industries. Downstream petroleum industry operations include the following categories: refinery, pipeline, marine, rail, bulk terminals and trucks, service stations, underground storage tanks, tank cleaning, and site characterization and remediation.
Exposure of the general population to benzene mainly occurs through breathing, the major sources of benzene being tobacco smoke (about 50%) as well as automobile service stations, exhaust from motor vehicles and industrial emissions (about 20% altogether). Vapors (or gases) from products that contain benzene, such as glues, paints, furniture wax, and detergents, can also be a source of exposure. The average smoker (32 cigarettes per day) takes in about 1.8 milligrams (mg) of benzene per day. This amount is about 10 times the average daily intake of benzene by nonsmokers.[41]
In 1987, OSHA estimated that about 237,000 workers in the United States were potentially exposed to benzene, but it is not known if this number has substantially changed since then.
Water and soil contamination are important pathways of concern for transmission of benzene contact. In the US alone, there are approximately 100,000 different sites that have benzene soil or groundwater contamination. In 2005, the water supply to the city of Harbin in China with a population of almost nine million people, was cut off because of a major benzene exposure. Benzene leaked into the Songhua River, which supplies drinking water to the city, after an explosion at a China National Petroleum Corporation (CNPC) factory in the city of Jilin on 13 November.
In March 2006, the official Food Standards Agency in Britain conducted a survey of 150 brands of soft drinks. It found that four contained benzene levels above World Health Organization limits. The affected batches were removed from sale.[42] (See also benzene in soft drinks).
Benzene exposure limits
The United States Environmental Protection Agency has set a maximum contaminant level (MCL) for benzene in drinking water at 0.005 mg/L (5 ppb), as promulgated via the National Primary Drinking Water Regulations.[43] This regulation is based on preventing benzene leukemogenesis. The maximum contaminant level goal (MCLG), a nonenforceable health goal that would allow an adequate margin of safety for the prevention of adverse effects, is zero benzene concentration in drinking water. The EPA requires that spills or accidental releases into the environment of 10 pounds (4.5 kg) or more of benzene be reported to the EPA.
The US Occupational Safety and Health Administration (OSHA) has set a permissible exposure limit of 1 part of benzene per million parts of air (1 ppm) in the workplace during an 8-hour workday, 40-hour workweek. The short term exposure limit for airborne benzene is 5 ppm for 15 minutes.[44] These legal limits were based on studies demonstrating compelling evidence of health risk to workers exposed to benzene. The risk from exposure to 1 ppm for a working lifetime has been estimated as 5 excess leukemia deaths per 1,000 employees exposed. (This estimate assumes no threshold for benzene's carcinogenic effects.) OSHA has also established an action level of 0.5 ppm to encourage even lower exposures in the workplace.[45]
The National Institute for Occupational Safety and Health (NIOSH) revised the Immediately Dangerous to Life or Health (IDLH) concentration for benzene to 500 ppm. The current NIOSH definition for an IDLH condition, as given in the NIOSH Respirator Selection Logic, is one that poses a threat of exposure to airborne contaminants when that exposure is likely to cause death or immediate or delayed permanent adverse health effects or prevent escape from such an environment [NIOSH 2004]. The purpose of establishing an IDLH value is (1) to ensure that the worker can escape from a given contaminated environment in the event of failure of the respiratory protection equipment and (2) is considered a maximum level above which only a highly reliable breathing apparatus providing maximum worker protection is permitted [NIOSH 2004[46]].[47] In September 1995, NIOSH issued a new policy for developing recommended exposure limits (RELs) for substances, including carcinogens. Because benzene can cause cancer, NIOSH recommends that all workers wear special breathing equipment when they are likely to be exposed to benzene at levels exceeding the REL (10-hour) of 0.1 ppm.[48] The NIOSH STEL (15 min) is 1 ppm.
American Conference of Governmental Industrial Hygienists (ACGIH) adopted Threshold Limit Values (TLVs) for benzene at 0.5 ppm TWA and 2.5 ppm STEL.
Under New Jersey's Right-to-Know law, respiratory protection for benzene is discussed.[49] As stated, improper use of respirators is dangerous. Respirators should only be used when there is a written respiratory program in place as described in the OSHA Respiratory Protection Standard (29 CFR 1910.134).[50] The employer is to develop and implement a written respiratory protection program with required worksite-specific procedures and elements for required respirator use. This program must be administered by a suitably trained program administrator. Employers must use the assigned protection factors (APF) listed in Table 1 of 29 CFR 1910.134 to select a respirator that meets or exceeds the required level of employee protection. For benzene:
- If there is a potential for exposure to 0.1 ppm, a NIOSH-approved half face respirator must be worn with an organic vapor cartridge (APF 10).
- If there is a potential for exposure to 0.5 ppm, a NIOSH-approved full face respirator must be worn with an organic vapor cartridge (APF 50).
- Where the potential exists for exposure to over 5 ppm, a NIOSH-approved air-supplied respirator with a full facepiece operated under pressure-demand or other positive-pressure mode must be used (APF 1,000).
Exposure monitoring
Airborne exposure monitoring for benzene must be conducted in order to properly assess personal exposures and effectiveness of engineering controls. Initial exposure monitoring should be conducted by an industrial hygienist or person specifically trained and experienced in sampling techniques. Contact an AIHA Accredited Laboratory for advice on sampling methods.[51]
Each employer with a place of employment where occupational exposures to benzene occur shall monitor each of these workplaces and work operations to determine accurately the airborne concentrations of benzene to which employees may be exposed.[52] Representative 8-hour TWA employee exposures need to be determined on the basis of one sample or samples representing the full shift exposure for each job classification in each work area. Unless air samples are taken frequently, the employer does not know the concentration and would not know how much of a protection factor is needed.[53]
In providing consultation on work safety during oil clean-up operations following the Deepwater Horizon accident, OSHA has worked with a number of other government agencies to protect Gulf cleanup workers. OSHA partnered with the NIOSH to issue "Interim Guidance for Protecting Deepwater Horizon Response Workers and Volunteers" and recommend measures that should be taken to protect workers from a variety of different health hazards that these workers face.[54] OSHA conceded that it recognizes that most of its PELs are outdated and inadequate measures of worker safety. In characterizing worker exposure, OSHA instead relies on more up-to-date recommended protective limits set by organizations such as NIOSH, the ACGIH, and the American Industrial Hygiene Association (AIHA), and not on the older, less protective PELS. Results of air monitoring are compared to the lowest known Occupational Exposure Limit for the listed contaminant for purposes of risk assessment and protective equipment recommendations. [55]
Biomarkers of exposure
Several tests can determine exposure to benzene. Benzene itself can be measured in breath, blood or urine, but such testing is usually limited to the first 24 hours post-exposure due to the relatively rapid removal of the chemical by exhalation or biotransformation. Most persons in developed countries have measureable baseline levels of benzene and other aromatic petroleum hydrocarbons in their blood. In the body, benzene is enzymatically converted to a series of oxidation products including muconic acid, phenylmercapturic acid, phenol, catechol, hydroquinone and 1,2,4-trihydroxybenzene. Most of these metabolites have some value as biomarkers of human exposure, since they accumulate in the urine in proportion to the extent and duration of exposure, and they may still be present for some days after exposure has ceased. The current ACGIH biological exposure limits for occupational exposure are 500 μg/g creatinine for muconic acid and 25 μg/g creatinine for phenylmercapturic acid in an end-of-shift urine specimen.[56][57][58][59]
Methods of excretion
Most inhaled benzene is not metabolized. Inhaled benzene is primarily expelled unchanged through exhalation. In a human study 16.4 to 41.6% of retained benzene was eliminated through the lungs within five to seven hours after a two- to three-hour exposure to 47 to 110 ppm and only 0.07 to 0.2% of the remaining benzene was excreted unchanged in the urine. After exposure to 63 to 405 mg/m3 of benzene for 1 to 5 hours, 51 to 87% was excreted in the urine as phenol over a period of 23 to 50 hours. In another human study, 30% of absorbed dermally applied benzene, which is primarily metabolized in the liver, was excreted as phenol in the urine.[60]
Molecular toxicology
The paradigm of toxicological assessment of benzene is slowly shifting towards the domain of molecular toxicology as it allows understanding of fundamental biological mechanisms in a better way. Glutathione seems to play an important role by protecting against benzene-induced DNA breaks and it is being identified as a new biomarker for exposure and effect.[61] Benzene causes chromosomal aberrations in the peripheral blood leukocytes and bone marrow explaining the higher incidence of leukemia and multiple myeloma caused by chronic exposure. These aberrations can be monitored using fluorescent in situ hybridization (FISH) with DNA probes to assess the effects of benzene along with the hematological tests as markers of hematotoxicity.[62] Benzene metabolism involves enzymes coded for by polymorphic genes. Studies have shown that genotype at these loci may influence susceptibility to the toxic effects of benzene exposure. Individuals carrying variant of NAD(P)H:quinone oxidoreductase 1 (NQO1), microsomal epoxide hydrolase (EPHX) and deletion of the glutathione S-transferase T1 (GSTT1) showed a greater frequency of DNA single-stranded breaks.[63]
Biological oxidation and carcinogenic activity
One way of understanding the carcinogenic effects of benzene is by examining the products of biological oxidation. Pure benzene, for example, oxidizes in the body to produce an epoxide, benzene oxide, which is not excreted readily and can interact with DNA to produce harmful mutations.
Summary
According to the Agency for Toxic Substances and Disease Registry (ATSDR) (2007), benzene is both an anthropogenically produced and naturally occurring chemical from processes that include: volcanic eruptions, wild fires, synthesis of chemicals such as phenol, production of synthetic fibers, and fabrication of rubbers, lubricants, pesticides, medications, and dyes. The major sources of benzene exposure are tobacco smoke, automobile service stations, exhaust from motor vehicles, and industrial emissions; however, ingestion and dermal absorption of benzene can also occur through contact with contaminated water. Benzene is hepatically metabolized and excreted in the urine. Measurement of air and water levels of benzene is accomplished through collection via activated charcoal tubes, which are then analyzed with a gas chromatograph. The measurement of benzene in humans can be accomplished via urine, blood, and breath tests; however, all of these have their limitations because benzene is rapidly metabolized in the human body into by-products called metabolites.[64]
OSHA regulates levels of benzene in the workplace.[65] The maximum allowable amount of benzene in workroom air during an 8-hour workday, 40-hour workweek is 1 ppm. Because benzene can cause cancer, NIOSH recommends that all workers wear special breathing equipment when they are likely to be exposed to benzene at levels exceeding the recommended (8-hour) exposure limit of 0.1 ppm.[66]
See also
References
- ^ Lide, D. R., ed (2005). CRC Handbook of Chemistry and Physics (86th ed.). Boca Raton (FL): CRC Press. ISBN 0-8493-0486-5.
- ^ Arnold, D.; Plank, C.; Erickson, E.; Pike, F. (1958). "Solubility of Benzene in Water.". Industrial & Engineering Chemistry Chemical & Engineering Data Series 3 (2): 253. doi:10.1021/i460004a016.
- ^ Breslow, R; Guo, T (1990). "Surface tension measurements show that chaotropic salting-in denaturants are not just water-structure breakers". Proceedings of the National Academy of Sciences of the United States of America 87 (1): 167–9. doi:10.1073/pnas.87.1.167. PMC 53221. PMID 2153285. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=53221.
- ^ A. Kayode Coker, Ernest E. Ludwig (2007). Ludwig's applied process design for chemical and petrochemical plants, Volume 1. Elsevier. p. 114. ISBN 075067766X. http://books.google.com/books?id=N8RcH8juG_YC&pg=PA114.
- ^ a b A. J. Rocke (1985). "Hypothesis and Experiment in the Early Development of Kekule's Benzene Theory". Annals of Science 42 (4): 355–81. doi:10.1080/00033798500200411.
- ^ M. Faraday (1825). "On New Compounds of Carbon and Hydrogen, and on Certain Other Products Obtained during the Decomposition of Oil by Heat". Philosophical Transactions of the Royal Society of London 115: 440–466. doi:10.1098/rstl.1825.0022. JSTOR 107752.
- ^ R. Kaiser (1968). "Bicarburet of Hydrogen. Reappraisal of the Discovery of Benzene in 1825 with the Analytical Methods of 1968". Angewandte Chemie International Edition in English 7 (5): 345–350. doi:10.1002/anie.196803451.
- ^ E. Mitscherlich (1834). "Über das Benzol und die Säuren der Oel- und Talgarten". Annalen der Pharmacie 9 (1): 39–48. doi:10.1002/jlac.18340090103.
- ^ August Kekulé: Ueber einige Condensationsproducte des Aldehyds, Liebigs Ann. Chem. 1872, 162 (1), 77–124, DOI:10.1002/jlac.18721620110.
- ^ F. A. Kekulé (1865). "Sur la constitution des substances aromatiques". Bulletin de la Societe Chimique de Paris 3: 98–110.
- ^ F. A. Kekulé (1866). "Untersuchungen uber aromatische Verbindungen". Liebigs Annalen der Chemie 137 (2): 129–36. doi:10.1002/jlac.18661370202.
- ^ English translation Wilcox, David H.; Greenbaum, Frederick R. (1965). "Kekule's benzene ring theory: A subject for lighthearted banter". Journal of Chemical Education 42 (5): 266–67. doi:10.1021/ed042p266.
- ^ F. A. Kekulé (1890). "Benzolfest: Rede". Berichte der Deutschen Chemischen Gesellschaft 23: 1302–11. doi:10.1002/cber.189002301204. http://gallica.bnf.fr/ark:/12148/bpt6k90720c/f1304.chemindefer.
- ^ Benfey O. T. (1958). "August Kekulé and the Birth of the Structural Theory of Organic Chemistry in 1858". Journal of Chemical Education 35: 21–23. doi:10.1021/ed035p21.
- ^ Jean Gillis, "Auguste Kekulé et son oeuvre, realisee a Gand de 1858 a 1867," Memoires de l'Academie Royale de Belgique, 37:1 (1866), 1–40.
- ^ K. Lonsdale (1929). "The Structure of the Benzene Ring in Hexamethylbenzene". Proceedings of the Royal Society 123A: 494.
- ^ K. Lonsdale (1931). "An X-Ray Analysis of the Structure of Hexachlorobenzene, Using the Fourier Method". Proceedings of the Royal Society 133A: 536–553. http://gallica.bnf.fr/ark:/12148/bpt6k56226p/f558.table.
- ^ bonding in benzene – the Kekulé structure
- ^ Moran, Damian; Simmonett, Andrew C.; Leach, Franklin E.; Allen, Wesley D.; Schleyer, Paul v. R.; Schaefer, Henry F. (2006). "Popular Theoretical Methods Predict Benzene and Arenes To Be Nonplanar". Journal of the American Chemical Society 128 (29): 9342. doi:10.1021/ja0630285. PMID 16848464.
- ^ Cooper, David L.; Gerratt, Joseph; Raimondi, Mario (1986). "The electronic structure of the benzene molecule". Nature 323 (6090): 699. doi:10.1038/323699a0.
- ^ Pauling, Linus (1987). "Electronic structure of the benzene molecule". Nature 325 (6103): 396. doi:10.1038/325396d0.
- ^ Messmer, Richard P.; Schultz, Peter A. (1987). "The electronic structure of the benzene molecule". Nature 329 (6139): 492. doi:10.1038/329492a0.
- ^ Harcourt, Richard D. (1987). "The electronic structure of the benzene molecule". Nature 329 (6139): 491. doi:10.1038/329491b0.
- ^ "Unicode Character 'BENZENE RING' (U+232C)". http://www.fileformat.info/info/unicode/char/232c/index.htm. Retrieved 2009-01-16.
- ^ "Unicode Character 'BENZENE RING WITH CIRCLE' (U+23E3)". http://www.fileformat.info/info/unicode/char/23e3/index.htm. Retrieved 2009-01-16.
- ^ Kolmetz, Gentry, Guidelines for BTX Revamps, AIChE 2007 Spring Conference
- ^ "Control of Hazardous Air Pollutants From Mobile Sources". U.S. Environmental Protection Agency. 2006-03-29. p. 15853. http://www.epa.gov/EPA-AIR/2006/March/Day-29/a2315b.htm. Retrieved 2008-06-27.
- ^ Stranks, D. R.; M. L. Heffernan, K. C. Lee Dow, P. T. McTigue, G. R. A. Withers (1970). Chemistry: A structural view. Carlton, Victoria: Melbourne University Press. p. 347. ISBN 0 522 83988 6.
- ^ Harrison's Principles of Internal Medicine, 16th ed., p. 618
- ^ Merck Manual, Home Edition, "Overview of Leukemia"
- ^ American Petroleum Institute, API Toxicological Review, Benzene, September 1948, Agency for Toxic Substances and Disease Registry, Department of Health and Human Services
- ^ WHO. INTERNATIONAL AGENCY FOR RESEARCH ON CANCER, IARC Monographs on the Evaluation of Carcinogenic Risks to Humans, Overall Evaluations of Carcinogenicity: An Updating of IARC Monographs, Volumes 1 to 42, Supplement 7
- ^ ToxFAQs for Benzene, Agency for Toxic Substances and Disease Registry, Department of Health and Human Services
- ^ ToxGuide for Benzene, Agency for Toxic Substances and Disease Registry, Department of Health and Human Services
- ^ OSHA Hazard Communication Standard 1910.1200(d)(5)(ii), carcinogens under (d)(4) of this section
- ^ 10/16/2003 – OSHA-recognized chemicals as carcinogens or potential carcinogens for Hazard Communication purposes. Osha.gov (2003-10-16). Retrieved on 2010-10-09.
- ^ Huff J (2007). "Benzene-induced cancers: abridged history and occupational health impact". Int J Occup Environ Health 13 (2): 213–21. PMID 17718179.
- ^ Rana SV; Verma Y (2005). "Biochemical toxicity of benzene". J Environ Biol 26 (2): 157–68. PMID 16161967.
- ^ Breathe carefully: air emissions of benzene may cause birth defects. — Environmental Health News. Environmentalhealthnews.org (2010-10-26). Retrieved on 2011-04-17.
- ^ Benzene exposure linked to sperm abnormalities that cause birth defects. — Environmental Health News. Environmentalhealthnews.org (2010-02-16). Retrieved on 2011-04-17.
- ^ Public Health Statement. Benzene, Division of Toxicology and Environmental Medicine, August 2007
- ^ "FDA: Too Much Benzene In Some Drinks", CBS News, May 19, 2006. Retrieved July 11, 2006.
- ^ Drinking Water Contaminants|Drinking Water Contaminants|US EPA. Water.epa.gov. Retrieved on 2010-10-09.
- ^ Chemical Sampling Information Benzene
- ^ Benzene Toxicity: Standards and Regulations|ATSDR – Environmental Medicine & Environmental Health Education – CSEM. Atsdr.cdc.gov (2000-06-30). Retrieved on 2010-10-09.
- ^ NIOSH respirator selection logic. Cincinnati, OH: U.S. Department of Health and Human Services, Public Health Service, Centers for Disease Control, National Institute for Occupational Safety and Health, DHHS (NIOSH). Publication No. 2005-100.
- ^ Documentation for Immediately Dangerous to Life or Health Concentrations (IDLH): Introduction
- ^ "Public Health Statement for Benzene" U.S. Department of Health and Human Services, Public Health Service, Centers for Disease Control, National Institute for Occupational Safety and Health
- ^ Benzene Fact Sheet
- ^ Respiratory Protection
- ^ Benzene. Product and Technical Information
- ^ Benzene. – 1910.1028. Osha.gov. Retrieved on 2010-10-09.
- ^ 01/10/1990 – Use of Air-Purifying Respirators In Dangerous Concentrations of Gases Or Vapors. Osha.gov. Retrieved on 2010-10-09.
- ^ NIOSH/OSHA Interim Guidance for Protecting Deepwater Horizon Response Workers and Volunteers, Osha.gov (2010-07-26)
- ^ Hazards Associated with Oil Cleanup Operations. Osha.gov (2010-07-02). Retrieved on 2010-10-09.
- ^ Ashley, DL; Bonin, MA; Cardinali, FL; McCraw, JM; Wooten, JV (1994). "Blood concentrations of volatile organic compounds in a nonoccupationally exposed US population and in groups with suspected exposure". Clinical chemistry 40 (7 Pt 2): 1401–4. PMID 8013127. http://www.clinchem.org/cgi/reprint/40/7/1401.pdf.
- ^ Fustinoni, S; Buratti, M; Campo, L; Colombi, A; Consonni, D; Pesatori, AC; Bonzini, M; Farmer, P et al. (2005). "Urinary t,t-muconic acid, S-phenylmercapturic acid and benzene as biomarkers of low benzene exposure". Chemico-biological interactions 153–154: 253–6. doi:10.1016/j.cbi.2005.03.031. PMID 15935823.
- ^ ACGIH. 2009 TLVs and BEIs. American Conference of Governmental Industrial Hygienists, Cincinnati, Ohio, 2009.
- ^ R. Baselt, Disposition of Toxic Drugs and Chemicals in Man, 8th edition, Biomedical Publications, Foster City, CA, 2008, pp. 144–148.
- ^ Benzene, CASRN: 71-43-2. Hazardous Substances Data Bank, U.S. National Library of Medicine. National Institutes of Health.
- ^ Fracasso, M.E.; Doria, D; Bartolucci, GB; Carrieri, M; Lovreglio, P; Ballini, A; Soleo, L; Tranfo, G et al. (2009). "Low air levels of benzene: Correlation between biomarkers of exposure and genotoxic effects". Toxicol Lett 192 (1): 22–8. doi:10.1016/j.toxlet.2009.04.028. PMID 19427373.
- ^ Eastmond, D.A.; Rupa, DS; Hasegawa, LS (2000). "Detection of hyperdiploidy and chromosome breakage in interphase human lymphocytes following exposure to the benzene metabolite hydroquinone using multicolor fluorescence in situ hybridization with DNA probes". Mutat Res 322 (1): 9–20. PMID 7517507.
- ^ Garte, S; Taioli, E; Popov, T; Bolognesi, C; Farmer, P; Merlo, F (2000). "Genetic susceptibility to benzene toxicity in humans". J Toxicol Environ Health A 71 (22): 1482–1489. doi:10.1080/15287390802349974. PMID 18836923.
- ^ Agency for Toxic Substances and Disease Registry. (2007). Benzene: Patient information sheet.
- ^ Occupational Safety and Health Standards, Toxic and Hazardous Substances, 1910.1028.
- ^ Public Health Statement for Benzene, Agency for Toxic Substances and Disease Registry. (August 2007). Benzene: Patient information sheet.
External links
- Benzene (www.eco-usa.net)
- International Chemical Safety Card 0015
- USEPA Summary of Benzene Toxicity
- NIOSH Pocket Guide to Chemical Hazards
- CID 241 from PubChem
- Dept. of Health and Human Services: TR-289: Toxicology and Carcinogenesis Studies of Benzene
- Video Podcast of Sir John Cadogan giving a lecture on Benzene since Faraday, in 1991
- Substance profile
- U.S. National Library of Medicine: ChemIDplus – Benzene
- NLM Hazardous Substances Databank – Benzene
Functional groups Acetyl · Acetoxy · Acryloyl · Acyl · Alcohol · Aldehyde · Alkane · Alkene · Alkyne · Alkoxy group · Amide · Amine · Azo compound · Benzene derivative · Carboxylic acid · Cyanate · Disulfide · Ester · Ether · Epoxide · Haloalkane · Hydrazone · Hydroxyl · Imine · Isocyanate · Isonitrile · Isothiocyanate · Ketone · Methine · Nitrile · Nitro compound · Nitroso compound · Organophosphorus · Oxime · Peroxide · Phosphonous and Phosphonic acid · Pyridine derivative · Sulfone · Sulfonic acid · Sulfoxide · Thiocyanate · Thioester · Thioether · Thiol · Urea
See also Chemical classification Categories:- Annulenes
- Simple aromatic rings
- IARC Group 1 carcinogens
- Soil contamination
- Hydrocarbon solvents
- Hazardous air pollutants
- Immunotoxins
- Occupational safety and health
Wikimedia Foundation. 2010.