- Cumene
-
Cumene 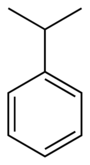
 (1-methylethyl)benzeneOther namesisopropylbenzene
(1-methylethyl)benzeneOther namesisopropylbenzene
2-phenylpropaneIdentifiers CAS number 98-82-8 
PubChem 7406 ChemSpider 7128 
UNII 8Q54S3XE7K 
KEGG C14396 
ChEBI CHEBI:34656 
RTECS number GR8575000 Jmol-3D images Image 1 - CC(C)c1ccccc1
Properties Molecular formula C9H12 Molar mass 120.19 g mol−1 Appearance colorless liquid Density 0.862 g cm−3, liquid Melting point −96 °C, 177 K, -141 °F
Boiling point 152 °C, 425 K, 306 °F
Solubility in water insoluble Viscosity 0.777 cP at 21 °C Hazards R-phrases R10,R37,R51/53,R65 S-phrases S24,S37,S61,S62 Main hazards flammable Flash point 43 °C Related compounds Related compounds ethylbenzene
toluene
benzene (verify) (what is:
(verify) (what is:  /
/ ?)
?)
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references Cumene is the common name for isopropylbenzene, an organic compound that is an aromatic hydrocarbon. It is a constituent of crude oil and refined fuels. It is a flammable colorless liquid that has a boiling point of 152 °C. Nearly all the cumene that is produced as a pure compound on an industrial scale is converted to cumene hydroperoxide, which is an intermediate in the synthesis of other industrially important chemicals, primarily phenol and acetone.
Contents
Production
Commercial production of cumene is carried out through the catalytic alkylation of benzene, with the addition of propylene. Solid phosphoric acid (SPA) supported on alumina can also be used as a catalyst, and this was the case prior to the mid-1990s when zeolite-based catalysts made the other technique commercially redundant. [1] This process is other wise known as Friedel-Crafts alkylation reaction.
Isopropylbenzene is stable, but may form peroxides in storage if in contact with the air. It is important to test for the presence of peroxides before heating or distilling. The chemical is also flammable and incompatible with strong oxidizing agents. Environmental laboratories commonly test isopropylbenzene using a Gas chromatography–mass spectrometry (GCMS) instrument.[2]
See also
References
- ^ The Innovation Group website, page accessed 15/11/07
- ^ http://www.caslab.com/Isopropylbenzene.php5
External links
Categories:- Hazardous air pollutants
- Alkylbenzenes
Wikimedia Foundation. 2010.
