- Cumulene
-
Not to be confused with cumene.
A cumulene is a chemical compound with two or more cumulative (consecutive) double bonds, for example butatriene (which is also just called cumulene), H2C=C=C=CH2. Unlike alkanes and most alkenes, cumulenes tend to be rigid, which makes them appealing for molecular nanotechnology. Polyynes are another kind of rigid carbon chains. Cumulenes are found in regions of space where hydrogen is rare. See astrochemistry.
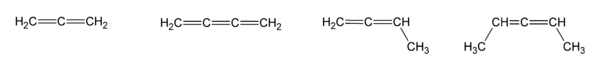
A collection of simple allenes and cumulenes. From left to right: propadiene (allene), butatriene (cumulene), buta-1,2-diene, penta-2,3-diene.
Inorganic cumulenes include carbon suboxide. An important class of organic cumulenes are ketenes, which are intermediate in character between CO2 and allene.
Contents
Structure
The rigidity of cumulenes arises from the fact that the internal carbon atoms carry two double bonds. Their sp hybridisation results in two bonds separated by 90°. Interestingly, cumulenes with (1) an even number of consecutive double bonds and (2) dissimilar substituents on either end can be chiral even though they lack a classical stereocenter. For example, penta-2,3-diene and hexa-1,3,4-triene are chiral. On the other hand odd numbers of double bonds in a cumulated system with proper substituents show cis-trans isomerism.
Reactions
The reactions of cumulene are those of the isolated double bond. Although this molecule possesses two π-bonds in very close vicinity, in essence these act as isolated double bonds. This is because the two π-bonds on the central carbon atom are formed by the non-hybridised p-orbitals. As these orbitals are perpendicular towards each other and occupy each other's nodal planes the two bonds are in essence isolated.
Transition metal cumulenes
The first reported complex containing a vinylidene ligand was (Ph2C2Fe2(CO)8, derived from the reaction of diphenylketene and Fe(CO)5 Structurally, this molecule resembles Fe2(CO)9, wherein one μ-CO ligand is replaced by 1,1-diphenylvinylidene, Ph2C2. The first monometallic vinylidene complex was (C5H5)Mo(P(C6H5)3)(CO)2[C=C(CN)2]Cl. [1]
See also
References
- ^ R. B. King (2004). "The Beginnings of Terminal Vinylidene Metal Complex Chemistry Through the Dicyanomethylene/Oxygen Analogy: Dicyanovinylidene Transition Metal Complexes". Coordination Chemistry Reviews 248 (15–16): 1533–1541. doi:10.1016/j.ccr.2004.05.003.
External links
- IUPAC Gold Book IUPACs definition of cumulenes

This organic chemistry article is a stub. You can help Wikipedia by expanding it.