- Covalent bond
-
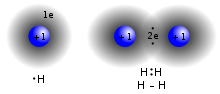
A covalent bond is a form of chemical bonding that is characterized by the sharing of pairs of electrons between atoms. The stable balance of attractive and repulsive forces between atoms when they share electrons is known as covalent bonding.[1]
Covalent bonding includes many kinds of interaction, including σ-bonding, π-bonding, metal-to-metal bonding, agostic interactions, and three-center two-electron bonds.[2][3] The term covalent bond dates from 1939.[4] The prefix co- means jointly, associated in action, partnered to a lesser degree, etc.; thus a "co-valent bond", in essence, means that the atoms share "valence", such as is discussed in valence bond theory. In the molecule H2, the hydrogen atoms share the two electrons via covalent bonding. Covalency is greatest between atoms of similar electronegativities. Thus, covalent bonding does not necessarily require the two atoms be of the same elements, only that they be of comparable electronegativity. Although covalent bonding entails sharing of electrons, it is not necessarily delocalized. Furthermore, in contrast to electrostatic interactions ("ionic bonds") the strength of covalent bond depends on the angular relation between atoms in polyatomic molecules.
Contents
History
 Early concepts in covalent bonding arose from this kind of image of the molecule of methane. Covalent bonding is implied in the Lewis structure that indicates sharing of electrons between atoms.
Early concepts in covalent bonding arose from this kind of image of the molecule of methane. Covalent bonding is implied in the Lewis structure that indicates sharing of electrons between atoms.
The term "covalence" in regard to bonding was first used in 1919 by Irving Langmuir in a Journal of the American Chemical Society article entitled "The Arrangement of Electrons in Atoms and Molecules". Langmuir wrote that "we shall denote by the term covalence the number of pairs of electrons which a given atom shares with its neighbors."[5]
The idea of covalent bonding can be traced several years before 1919 to Gilbert N. Lewis, who in 1916 described the sharing of electron pairs between atoms.[6] He introduced the Lewis notation or electron dot notation or Lewis dot structure in which valence electrons (those in the outer shell) are represented as dots around the atomic symbols. Pairs of electrons located between atoms represent covalent bonds. Multiple pairs represent multiple bonds, such as double and triple bonds. Some examples of Electron Dot Notation are shown in the following figure. An alternative form of representation, not shown here, has bond-forming electron pairs represented as solid lines. While the idea of shared electron pairs provides an effective qualitative picture of covalent bonding, quantum mechanics is needed to understand the nature of these bonds and predict the structures and properties of simple molecules. Walter Heitler and Fritz London are credited with the first successful quantum mechanical explanation of a chemical bond, specifically that of molecular hydrogen, in 1927.[7] Their work was based on the valence bond model, which assumes that a chemical bond is formed when there is good overlap between the atomic orbitals of participating atoms. These atomic orbitals are known to have specific angular relationships between each other, and thus the valence bond model can successfully predict the bond angles observed in simple molecules.
Physical properties of covalent compounds (polar/non-polar)
Physical properties Covalent compounds States (at room temperature) Solid, liquid, gas Electrical conductivity Usually none Boiling point and Melting point Varies, but usually lower than ionic compounds Solubility in water Varies, but usually lower than ionic compounds Thermal conductivity Usually low Polarity of covalent bonds
Covalent bonds are affected by the electronegativity of the connected atoms. Two atoms with equal electronegativity will make non-polar covalent bonds such as H-H. An unequal relationship creates a polar covalent bond such as with H-Cl.
Subdivision of covalent bonds
There are three types of covalent substances: individual molecules, molecular structures, and macromolecular structures. Individual molecules have strong bonds that hold the atoms together, but there are negligible forces of attraction between molecules. Such covalent substances are gases. For example, HCl, SO2, CO2, and CH4. In molecular structures, there are weak forces of attraction. Such covalent substances are low-boiling-temperature liquids (such as ethanol), and low-melting-temperature solids (such as iodine and solid CO2). Macromolecular structures have large numbers of atoms linked in chains or sheets (such as graphite), or in 3-dimensional structures (such as diamond and quartz). These substances have high melting and boiling points, are frequently brittle, and tend to have high electrical resistivity. Elements that have high electronegativity, and the ability to form three or four electron pair bonds, often form such large macromolecular structures.[8]
See also
- Metallic bonding
- Bonding in solids
- Linear combination of atomic orbitals
- Hybridization
- Hydrogen bond
- Noncovalent bonding
- Disulfide bond
- Ionic bond
- Covalent radius
- Resonance (chemistry)
- Bond order
References
- ^ Campbell, Neil A.; Brad Williamson; Robin J. Heyden (2006). Biology: Exploring Life. Boston, Massachusetts: Pearson Prentice Hall. ISBN 0-13-250882-6. http://www.phschool.com/el_marketing.html.
- ^ March, J. “Advanced Organic Chemistry” 4th Ed. J. Wiley and Sons, 1991: New York. ISBN 0-471-60180-2.
- ^ G. L. Miessler and D. A. Tarr “Inorganic Chemistry” 3rd Ed, Pearson/Prentice Hall publisher, ISBN 0-13-035471-6.
- ^ Merriam-Webster – Collegiate Dictionary (2000).
- ^ Langmuir, Irving (1919-06-01). "The Arrangement of Electrons in Atoms and Molecules". Journal of the American Chemical Society 41 (6): 868–934. doi:10.1021/ja02227a002.
- ^ Lewis, Gilbert N. (1916-04-01). "The atom and the molecule". Journal of the American Chemical Society 38 (4): 762–785. doi:10.1021/ja02261a002.
- ^ W. Heitler and F. London, Zeitschrift für Physik, vol. 44, p. 455 (1927). English translation in Hinne Hettema (2000). Quantum chemistry: classic scientific papers. World Scientific. pp. 140–. ISBN 978-981-02-2771-5. http://books.google.com/books?id=qsidHRJmUoIC&pg=140. Retrieved 3 November 2011.
- ^ Stranks, D. R.; M. L. Heffernan, K. C. Lee Dow, P. T. McTigue, G. R. A. Withers (1970). Chemistry: A structural view. Carlton, Victoria: Melbourne University Press. p. 184. ISBN 0 522 83988 6.
Sources
- "Covalent bonding — Single bonds". chemguide. 2000. http://www.chemguide.co.uk/atoms/bonding/covalent.html.
- "Electron Sharing and Covalent Bonds". Department of Chemistry University of Oxford. http://www.chem.ox.ac.uk/vrchemistry/electronsandbonds/intro1.htm.
- "Chemical Bonds". Department of Physics and Astronomy, Georgia State University. http://hyperphysics.phy-astr.gsu.edu/hbase/chemical/bond.html#c5.
External links
Chemical bonds Intramolecular
("strong")Covalent bondsSigma bond · Pi bond · Delta bond
Double bond · Triple bond · Quadruple bond · Quintuple bond · Sextuple bond
3c–2e · 3c–4e · 4c–2e
Agostic bond · Bent bond · Dipolar bond · Pi backbond
Conjugation · Hyperconjugation · Aromaticity · Hapticity · AntibondingCation–pi bond · Salt bondIntermolecular
("weak")Other noncovalentNote: the weakest strong bonds are not necessarily stronger than the strongest weak bondsConcepts in organic chemistry Aromaticity · Covalent bonding · Functional groups · Nomenclature · Organic compounds · Organic reactions · Organic synthesis · Publications · Spectroscopy · Stereochemistry · List of organic compoundsCategories:- Chemical bonding
Wikimedia Foundation. 2010.