- Delta bond
-
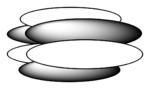
In chemistry, delta bonds (δ bonds) are chemical bonds of the covalent type, where four lobes of one involved atomic orbital overlap four lobes of the other involved atomic orbital. This overlap leads to the formation of a bonding molecular orbital with two nodal planes which contain the internuclear axis and go through both atoms.
The Greek letter δ in their name refers to d orbitals, since the orbital symmetry of the delta bond is the same as that of the usual (4-lobed) type of d orbital when seen down the bond axis.
In chemistry, in sufficiently large atoms, occupied d-orbitals are low enough in energy to participate in bonding. Delta bonds are usually observed in organometallic species. Some ruthenium and molybdenum compounds contain a quadruple bond, consisting of one sigma bond, two pi bonds and one delta bond.
The orbital symmetry of the delta bonding orbital is different from that of a pi antibonding orbital, which has one nodal plane containing the internuclear axis and a second nodal plane perpendicular to this axis between the atoms.
Theoretical chemists have conjectured that higher-order bonds (phi bonds and gamma bonds, corresponding to overlap of f and g orbitals) are possible, with even more overlapping lobes of their component atomic orbitals. There is as of the time of writing only one known example of a molecule purported to contain a phi bond (a U−U bond, in the molecule U2).[1]
See also
References
- F.A. Cotton and G. Wilkinson, "Advanced Inorganic Chemistry" (5th edn, John Wiley 1988), p. 1087-1091
- B. Douglas, D.H. McDaniel and J.J. Alexander, "Concepts and Models of Inorganic Chemistry" (2nd edn, Wiley 1983), p. 137
- J.E. Huheey, "Inorganic Chemistry" (3d edn, Harper and Row 1983). p. 743-744
- G.L. Miessler and D.A. Tarr, "Inorganic Chemistry" (2nd edn, Prentice-Hall 1999), p. 123-124
- ^ Gagliardi, Laura; Roos, Björn O. (2005). "Quantum chemical calculations show that the uranium molecule U2 has a quintuple bond". Nature 433: 848–851. doi:http://dx.doi.org/10.1038/nature03249. http://archive-ouverte.unige.ch/unige:3652.
Chemical bonds Intramolecular
("strong")Sigma bond · Pi bond · Delta bond
Double bond · Triple bond · Quadruple bond · Quintuple bond · Sextuple bond
3c–2e · 3c–4e · 4c–2e
Agostic bond · Bent bond · Dipolar bond · Pi backbond
Conjugation · Hyperconjugation · Aromaticity · Hapticity · AntibondingCation–pi bond · Salt bondIntermolecular
("weak")Other noncovalentNote: the weakest strong bonds are not necessarily stronger than the strongest weak bondsCategories:- Chemical bonding
Wikimedia Foundation. 2010.