- Intermolecular force
-
Intermolecular forces (forces between two molecules) are weak compared to the intramolecular forces (forces keeping a molecule together). For example, the covalent bond present within HCl molecules is much stronger than the forces present between the neighbouring molecules. These forces exist between molecules when they are sufficiently close to each other. The forces consist of four types:
- Dipole–dipole forces
- Ion–dipole forces
- Dipole-induced dipole force or Debye forces
- Instantaneous dipole-induced dipole forces or London dispersion forces.
Contents
London dispersion forces
Main article: London dispersion forceOtherwise known as quantum-induced instantaneous polarization or instantaneous dipole-induced dipole forces, the London dispersion force is caused by correlated movements of the electrons in interacting molecules. The electrons, which belong to different molecules, start "feeling" and avoiding each other at the short intermolecular distances, which is frequently described as formation of "instantaneous dipoles" that attract each other.
Debye (induced dipole) force
The induced dipole forces appear from the induction (also known as polarization), which is the attractive interaction between a permanent multipole on one molecule with an induced (by the former di/multi-pole) multipole on another.[1][2][3][4] This interaction is called Debye force after Peter J.W. Debye.
The example of an induction-interaction between permanent dipole and induced dipole is HCl and Ar. In this system, Ar experiences a dipole as its electrons are attracted (to H side) or repelled (from Cl side) by HCl.[1][3] This kind of interaction can be expected between any polar molecule and non-polar/symmetrical molecule. The induction-interaction force is far weaker than dipole-dipole interaction, however stronger than London force.They induce their properties in another atom
Dipole–dipole interactions
Dipole–dipole interactions are electrostatic interactions of permanent dipoles in molecules. These interactions tend to align the molecules to increase the attraction (reducing potential energy). An example of a dipole–dipole interaction can be seen in hydrogen chloride (HCl): The positive end of a polar molecule will attract the negative end of the other molecule and cause them to be arranged in a specific arrangement. Polar molecules have a net attraction between them. For example HCl and chloroform (CHCl3)
Keesom interactions (named after Willem Hendrik Keesom) are attractive interactions of dipoles that are Boltzmann-averaged over different rotational orientations of the dipoles. The energy of a Keesom interaction depends on the inverse sixth power of the distance, unlike the interaction energy of two spatially fixed dipoles, which depends on the inverse third power of the distance.
Often, molecules have dipolar groups within them, but have no overall dipole moment. This occurs if there is symmetry within the molecule, causing the dipoles to cancel each other out. This occurs in molecules such as tetrachloromethane. Note that the dipole–dipole interaction between two atoms is usually zero, because atoms rarely carry a permanent dipole. See atomic dipoles.
Ion-Dipole and Ion-Induced Dipole Forces
Ion-dipole and induced-dipole forces operate much like dipole-dipole and induced-dipole interactions. However, instead of only polar and non-polar molecules being involved, instead, ion interactions involve ions (as the name suggests). Ion-dipole and ion-induced dipole forces are stronger than dipole interactions because the charge of any ion is much greater than the charge of a dipole movement. Of course, H-bonds (a form of dipole-dipole) are still stronger than ion interactions.
An ion-dipole force consists of an ion and a polar molecule interacting. They align so that the positive and negative forces are next to one another, allowing for maximum attraction.
An ion-induced dipole force consists of an ion and a non-polar molecule interacting. Like a dipole-induced dipole force, the charge of the ion causes a distortion of the electron cloud on the non-polar molecule. [5]
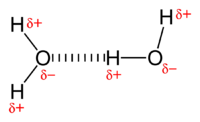
Hydrogen bonding
Main article: Hydrogen bondA hydrogen bond is the attractive force between an electronegative atom and a hydrogen atom that is bonded to either nitrogen, oxygen, or fluorine.[6] The hydrogen bond is often described as a strong electrostatic dipole–dipole interaction. However, it also has some features of covalent bonding: It is directional, stronger than a van der Waals interaction, produces interatomic distances shorter than sum of van der Waals radii, and usually involves a limited number of interaction partners, which can be interpreted as a kind of valence.
Intermolecular hydrogen bonding is responsible for the high boiling point of water (100 °C) compared to the other group 16 hydrides that have no hydrogen bonds. Intramolecular hydrogen bonding is partly responsible for the secondary, tertiary, and quaternary structures of proteins and nucleic acids. It also plays an important role in the structure of polymers, both synthetic and natural.
Relative strength of forces
Bond type Dissociation energy (kcal),[7][8] Covalent 400 Hydrogen bonds 12–16 Dipole–dipole 0.5–2 London (van der Waals) Forces <1 Note: this comparison is only approximate – the actual relative strengths will vary depending on the molecules involved.
Quantum mechanical theories
Main article: Quantum mechanical explanation of intermolecular interactionsSee also
- Coomber's relationship
- Force field
- Hydrophobic effect
- Intramolecular force
- Molecular solid
- Polymer
- Quantum chemistry computer programs
- Software for molecular mechanics modeling
References
- ^ a b Blustin PH, 1978. A Floating Gaussian Orbital calculation on argon hydrochloride (Ar • HCl). Theoret. Chim. Acta 47, 249–257.
- ^ Nannoolal Y, 2006. Development and critical evaluation of group contribution methods for the estimation of critical properties, liquid vapour pressure and liquid viscosity of organic compounds. University of Kwazulu-Natal PhD Thesis.
- ^ a b Roberts JK and Orr WJC, 1938. Induced dipoles and the heat of adsorption of argon on ionic crystals. Trans. Faraday Soc. 34, 1346–1349.
- ^ Sapse AM, Rayez-Meaume MT, Rayez JC and Massa LJ, 1979. Ion-induced dipole H-n clusters. Nature 278, 332–333.
- ^ Dr. Michael Blaber, 1996. Intermolecular Forces. http://www.mikeblaber.org/oldwine/chm1045/notes/Forces/Intermol/Forces02.htm
- ^ IUPAC, Compendium of Chemical Terminology, 2nd ed. (the "Gold Book") (1997). Online corrected version: (2006–) "hydrogen bond".
- ^ Volland, Dr. Walt. ""Intermolecular" Forces". http://www.800mainstreet.com/08/0008-0012-interforce.html. Retrieved 2009-09-20.
- ^ Organic Chemistry: Structure and Reactivity by Seyhan Ege, pp.30–33, 67
External links
- Software for calculation of intermolecular forces
- Quantum 3.2
- SAPT: An ab initio quantumchemical package.
Chemical bonds Intramolecular
("strong")Sigma bond · Pi bond · Delta bond
Double bond · Triple bond · Quadruple bond · Quintuple bond · Sextuple bond
3c–2e · 3c–4e · 4c–2e
Agostic bond · Bent bond · Dipolar bond · Pi backbond
Conjugation · Hyperconjugation · Aromaticity · Hapticity · AntibondingCation–pi bond · Salt bondIntermolecular
("weak")Other noncovalentNote: the weakest strong bonds are not necessarily stronger than the strongest weak bondsCategories:- Chemical bonding
- Intermolecular forces
Wikimedia Foundation. 2010.