- Double bond
-
A double bond in chemistry is a chemical bond between two chemical elements involving four bonding electrons instead of the usual two. The most common double bond, that between two carbon atoms, can be found in alkenes. Many types of double bonds between two different elements exist, for example in a carbonyl group with a carbon atom and an oxygen atom. Other common double bonds are found in azo compounds (N=N), imines (C=N) and sulfoxides (S=O). In skeletal formula the double bond is drawn as two parallel lines (=) between the two connected atoms; typographically, the equals sign is used for this.[1][2]
Double bonds are stronger than single bonds and double bonds are also shorter. The bond order is two. Double bonds are also electron-rich, which makes them reactive.
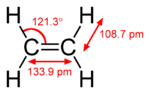
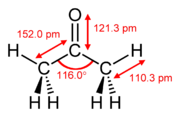
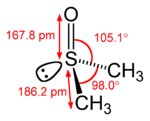
ethylene acetone dimethyl sulfoxide Common chemical compounds with double bonds Contents
Bonding
The type of bonding can be explained in terms of orbital hybridization. In ethylene each carbon atom has three sp2 orbitals and one p-orbital. The three sp2 orbitals lie in a plane with 120° angles. The p-orbital is perpendicular to this plane. When the carbon atoms approach each other, two of the sp2 orbitals overlap to form a sigma bond. At the same time, the two p-orbitals approach (again in the same plane) and together they form a pi-bond. For maximum overlap, the p-orbitals have to remain parallel, and, therefore, rotation around the central bond is not possible. This property gives rise to cis-trans isomerism. Double bonds are shorter than single bonds because p-orbital overlap is maximized.
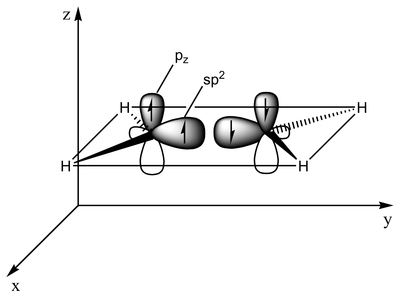
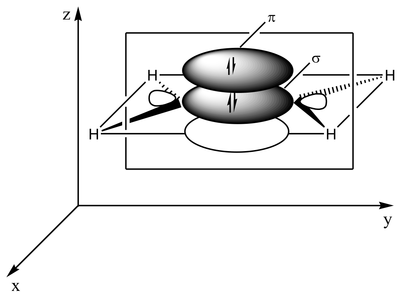
2 sp2 orbitals (total of 3 such orbitals) approach to form a sp2-sp2 sigma bond Two p-orbitals overlap to form a pi-bond in a plane parallel to the sigma plane
With 133 pm, the C−C bond length is shorter than the C−C length in ethane with 154 pm. The double bond is also stronger, 636 (KJ/mol) versus 368 kJ/mole but not twice as much as the pi-bond is weaker than the sigma bond due to less effective pi-overlap.In an alternative representation, the double bond results from two overlapping sp3 orbitals as in a bent bond.[3]
Types of double bonds between atoms
C O N S C alkene carbonyl group imine thioketone, thial O dioxygen nitroso compound sulfoxide, sulfone, sulfinic acid, sulfonic acid N azo compound S disulfur Variations
In molecules with alternating double bonds and single bonds, p-orbital overlap can exist over multiple atoms in a chain, giving rise to a conjugated system. Conjugation can be found in systems such as dienes and enones. In cyclic molecules, conjugation can lead to aromaticity. In cumulenes two double bonds are adjacent.
Double bonds are common for period 2 elements carbon, nitrogen, and oxygen, and less common with elements of higher periods. Metals, too, can engage in multiple bonding in a metal ligand multiple bond.
References
- ^ March, Jerry (1985), Advanced Organic Chemistry: Reactions, Mechanisms, and Structure (3rd ed.), New York: Wiley, ISBN 0-471-85472-7
- ^ Organic Chemistry 2nd Ed. John McMurry
- ^ Advanced Organic Chemistry Carey, Francis A., Sundberg, Richard J. 5th ed. 2007
Chemical bonds Intramolecular
("strong")Sigma bond · Pi bond · Delta bond
Double bond · Triple bond · Quadruple bond · Quintuple bond · Sextuple bond
3c–2e · 3c–4e · 4c–2e
Agostic bond · Bent bond · Dipolar bond · Pi backbond
Conjugation · Hyperconjugation · Aromaticity · Hapticity · AntibondingCation–pi bond · Salt bondIntermolecular
("weak")Other noncovalentNote: the weakest strong bonds are not necessarily stronger than the strongest weak bonds Categories:- Chemical bonding
Wikimedia Foundation. 2010.
