- Acetone
-
Acetone[1] 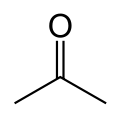
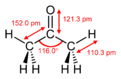
 Systematic namePropan-2-one[2]
Systematic namePropan-2-one[2]Identifiers Abbreviations DMK CAS number 67-64-1 
PubChem 180 ChemSpider 175 
UNII 1364PS73AF 
EC number 200-662-2 UN number 1090 KEGG D02311 
MeSH Acetone ChEBI CHEBI:15347 
ChEMBL CHEMBL14253 
RTECS number AL3150000 Beilstein Reference 635680 Gmelin Reference 1466 3DMet B00058 Jmol-3D images Image 1 - CC(C)=O
Properties Molecular formula C3H6O Molar mass 58.08 g mol−1 Exact mass 58.041864814 g mol−1 Appearance Colorless liquid Odor Pungent, irritating, floral Density 0.791 g cm−3[6] Melting point -95--93 °C, 178-180 K, -139--136 °F
Boiling point 56-57 °C, 329-330 K, 133-134 °F
log P -0.042 Vapor pressure 24.46-24.60 kPa (at 20 °C) Acidity (pKa) 24.2 Basicity (pKb) -10.2 Refractive index (nD) 1.35900 Viscosity 0.3075 cP Structure Coordination
geometryTriagonal planar at C2 Molecular shape Dihedral at C2 Dipole moment 2.91 D Thermochemistry Std enthalpy of
formation ΔfHo298-250.03-(-248.77) kJ mol−1 Std enthalpy of
combustion ΔcHo298-1.772 MJ mol−1 Standard molar
entropy So298200.4 J K−1 mol−1 Specific heat capacity, C 125.45 J K−1 mol−1 Hazards MSDS External MSDS GHS pictograms 

GHS signal word DANGER GHS hazard statements H225, H319, H336 GHS precautionary statements P210, P261, P305+351+338 EU Index 606-001-00-8 EU classification  F
F  Xi
XiR-phrases R11, R36, R66, R67 S-phrases (S2), S9, S16, S26 NFPA 704 Flash point −17 °C Autoignition
temperature465 °C Explosive limits 13.2–57.0% Threshold Limit Value 500 ppm (TWA), 750 ppm (STEL) LD50 >2000 mg/kg, oral (rat) Related compounds Related compounds Butanone
Isopropanol
Supplementary data page Structure and
propertiesn, εr, etc. Thermodynamic
dataPhase behaviour
Solid, liquid, gasSpectral data UV, IR, NMR, MS  (verify) (what is:
(verify) (what is:  /
/ ?)
?)
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references Acetone is the organic compound with the formula (CH3)2CO, a colorless, mobile, flammable liquid, the simplest example of the ketones.
Acetone is miscible with water and serves as an important solvent in its own right, typically as the solvent of choice for cleaning purposes in the laboratory. About 6.7 million tonnes were produced worldwide in 2010, mainly for use as a solvent and production of methyl methacrylate and bisphenol A.[7][8] Familiar household uses of acetone are as the active ingredient in nail polish remover and as paint thinner. It is a common building block in organic chemistry.
Acetone is naturally produced and disposed of in the human body as a result of normal metabolic processes. It is normally present in blood and urine. Diabetic people produce it in larger amounts. Reproductive toxicity tests show that it has low potential to cause reproductive problems. In fact, the body naturally increases the level of acetone in pregnant women, nursing mothers and children because their higher energy requirements lead to higher levels of acetone production. Ketogenic diets that increase acetone in the body are used to reduce epileptic attacks in infants and children who suffer from recalcitrant refractory epilepsy.
Contents
Production
In 2010, the worldwide production capacity for acetone was estimated at 6.7 million tonnes per year.[9] With 1.56 million tonnes per year, the United States had the highest production capacity,[10] followed by Taiwan and mainland China. The largest producer of acetone is INEOS Phenol, owning 17% of the world's capacity, with also significant capacity (7-8%) by Mitsui, Sunoco and Shell in 2010.[9] INEOS Phenol also owns the world's largest production site (420,000 tonnes/annum) in Beveren (Belgium). The price of acetone varied in 2010 between $75 and $110/tonne in the United States and $90 and 100 in Western Europe.[9]
Production Process
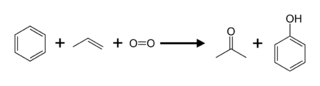
Acetone is produced directly or indirectly from propylene. Approximately 83 % of acetone is produced via the cumene process,[8] as a result, acetone production is tied to phenol production. In the cumene process, benzene is alkylated with propylene and the resulting cumene (isopropylbenzene) is oxidized by air to give phenol and acetone:
Other processes involve the direct oxidation of propylene (Wacker-Hoechst process), or the hydration of propylene to give 2-propanol, which is oxidized to acetone.[8]
Older production methods
Previously, acetone was produced by the dry distillation of acetates, for example calcium acetate. During World War I acetone was produced via bacterial fermentation, as developed by Chaim Weizmann (later the first president of Israel) in order to help the British war effort.[8] This Acetone Butanol Ethanol process was abandoned due to the small yields.[8]
Biosynthesis
See also: ketosisSmall amounts of acetone are produced in the body by the decarboxylation of ketone bodies. Since it is a byproduct of fermentation, acetone is a byproduct of the distillery industry.
Uses
About a third of the world's acetone is used as a solvent, and a quarter is consumed as a precursor to methyl methacrylate.[7]
Solvent use
Acetone is a good solvent for most plastics and synthetic fibers including those used in laboratory bottles made of polystyrene, polycarbonate and some types of polypropylene.[11] It is ideal for thinning fiberglass resin, cleaning fiberglass tools and dissolving two-part epoxies and superglue before hardening. It is used as a volatile component of some paints and varnishes. As a heavy-duty degreaser, it is useful in the preparation of metal prior to painting; it also thins polyester resins, vinyl and adhesives. It is also useful for high reliability soldering applications to remove solder rosin after soldering is complete. This helps to prevent the Rusty bolt effect from occurring due to dirty solder contacts.
Many millions of kilograms of acetone are consumed in the production of the solvents methyl isobutyl alcohol and methyl isobutyl ketone. These products arise via an initial aldol condensation to give diacetone alcohol.[8]
- 2 (CH3)2CO → (CH3)2C(OH)CH2C(O)CH3
Acetone is used as a solvent by the pharmaceutical industry and as a denaturation agent in denatured alcohol.[12] Acetone is also present as an excipient in some pharmaceutical products.[13]
Storage of acetylene
Although flammable itself, acetone is also used extensively as a solvent for the safe transporting and storing of acetylene, which cannot be safely pressurized as a pure compound. Vessels containing a porous material are first filled with acetone followed by acetylene, which dissolves into the acetone. One liter of acetone can dissolve around 250 liters of acetylene.[14][15]
Methyl methacrylate
This application begins with the initial conversion of acetone to acetone cyanohydrin:
- (CH3)2CO + HCN → (CH3)2C(OH)CN
In a subsequent step, the nitrile is hydrolyzed to the unsaturated amide, which is esterified:
- (CH3)2C(OH)CN + CH3OH → CH2=(CH3)CCO2CH3 + NH3
The third major use of acetone (about 20%)[7] entails its condensation with phenol to give bisphenol A:
- (CH3)2CO + 2 C6H5OH → (CH3)2C(C6H4OH)2 + H2O
Bisphenol A is a component of many polymers such as polycarbonates, polyurethanes, and epoxy resins.
Medical and cosmetic uses
Acetone is used in a variety of general medical and cosmetic applications and is also listed as a component in food additives and food packaging.
Acetone is commonly used in chemical peeling. Common agents used today for chemical peels are salicylic acid, lycolic acid, 30% salicylic acid in ethanol, and trichloroacetic acid (TCA). Prior to chemexfoliation, the skin should be cleaned properly and excess fat removed. This process is known as defatting. Acetone, Septisol, or a combination of these agents is commonly used in this process.[citation needed]
Laboratory uses
In the laboratory, acetone is used as a polar aprotic solvent in a variety of organic reactions, such as SN2 reactions. The use of acetone solvent is also critical for the Jones oxidation. It is a common solvent for rinsing laboratory glassware because of its low cost and volatility. However, it does not form an azeotrope with water (see azeotrope (data)).[16] Despite its common use as a supposed drying agent, it is not effective except by bulk displacement and dilution. Acetone can be cooled with dry ice to −78 °C without freezing; acetone/dry ice baths are commonly used to conduct reactions at low temperatures. Acetone is fluorescent under ultraviolet light, and its vapor may be used as a fluorescent tracer in fluid flow experiments.[17]
Domestic and other niche uses
Acetone is often the primary component in cleaning agents such as nail polish remover. Ethyl acetate, another organic solvent, is sometimes used as well. Acetone is a component of superglue remover and it easily removes residues from glass and porcelain.
It can be used as an artistic agent; when rubbed on the back of a laser print or photocopy placed face-down on another surface and burnished firmly, the toner of the image transfers to the destination surface.
Make-up artists use acetone to remove skin adhesive from the netting of wigs and moustaches by immersing the item in an acetone bath, then removing the softened glue residue with a stiff brush.
Safety
Flammability
The most common hazard associated with acetone is its extreme flammability. It auto-ignites at a temperature of 465 °C (869 °F). At temperatures greater than acetone's flash point of −20 °C (−4 °F), air mixtures of between 2.5% and 12.8% acetone, by volume, may explode or cause a flash fire. Vapors can flow along surfaces to distant ignition sources and flash back. Static discharge may also ignite acetone vapors.[18]
Health information
Acetone has been studied extensively and is generally recognized to have low acute and chronic toxicity if ingested and/or inhaled. Inhalation of high concentrations (around 9200 ppm) in the air caused irritation of the throat in humans in as little as 5 minutes. Inhalation of concentrations of 1000 ppm caused irritation of the eyes and of the throat in less than 1 hour; however, the inhalation of 500 ppm of acetone in the air caused no symptoms of irritation in humans even after 2 hours of exposure. Acetone is not currently regarded as a carcinogen, a mutagenic chemical or a concern for chronic neurotoxicity effects.[18]
Acetone can be found as an ingredient in a variety of consumer products ranging from cosmetics to processed and unprocessed foods. Acetone has been rated as a GRAS (Generally Recognized as Safe) substance when present in beverages, baked foods, desserts, and preserves at concentrations ranging from 5 to 8 mg/L. Additionally, a joint U.S-European study found that acetone’s "health hazards are slight."[citation needed]
Toxicology
Acetone is believed to exhibit only slight toxicity in normal use, and there is no strong evidence of chronic health effects if basic precautions are followed.[19]
At very high vapor concentrations, acetone is irritating and, like many other solvents, may depress the central nervous system. It is also a severe irritant on contact with eyes, and a potential pulmonary aspiration risk. In one documented case, ingestion of a substantial amount of acetone led to systemic toxicity, although the patient eventually fully recovered.[20] Some sources estimate LD50 for human ingestion at 1.159 g/kg; LD50 inhalation by mice is given as 44 g/m3, over 4 hours.[21]
Acetone has been shown to have anticonvulsant effects in animal models of epilepsy, in the absence of toxicity, when administered in millimolar concentrations.[22] It has been hypothesized that the high-fat low-carbohydrate ketogenic diet used clinically to control drug-resistant epilepsy in children works by elevating acetone in the brain.[22]
- EPA EPCRA Delisting (1995). EPA removed acetone from the list of “toxic chemicals” maintained under Section 313 of the Emergency Planning and Community Right to Know Act (EPCRA). In making that decision, EPA conducted an extensive review of the available toxicity data on acetone and found that acetone "exhibits acute toxicity only at levels that greatly exceed releases and resultant exposures", and further that acetone "exhibits low toxicity in chronic studies".
- Genotoxicity. Acetone has been tested in more than two dozen in vitro and in vivo assays. These studies indicate that acetone is not genotoxic.
- Carcinogenicity. EPA in 1995 concluded, "There is currently no evidence to suggest a concern for carcinogenicity". (EPCRA Review, described in Section 3.3). NTP scientists have recommended against chronic toxicity/carcinogenicity testing of acetone because "the prechronic studies only demonstrated a very mild toxic response at very high doses in rodents".
- Neurotoxicity and Developmental Neurotoxicity. The neurotoxic potential of both acetone and isopropanol, the metabolic precursor of acetone, have been extensively studied. These studies demonstrate that although exposure to high doses of acetone may cause transient central nervous system effects, acetone is not a neurotoxicant. A guideline developmental neurotoxicity study has been conducted with isopropanol, and no developmental neurotoxic effects were identified, even at the highest dose tested. (SIAR, pp. 1, 25, 31).
- Environmental. When the EPA exempted acetone from regulation as a volatile organic compound (VOC) in 1995, EPA stated that this exemption would "contribute to the achievement of several important environmental goals and would support EPA’s pollution prevention efforts". 60 Fed. Reg. 31,634 (June 16, 1995). 60 Fed. Reg. 31,634 (June 16, 1995). EPA noted that acetone could be used as a substitute for several compounds that are listed as hazardous air pollutants (HAP) under section 112 of the [Clean Air] Act.
Environmental effects
Acetone evaporates rapidly, even from water and soil. Once in the atmosphere, it is degraded by UV light with a 22-day half-life. Acetone dissipates slowly in soil, animals, or waterways since it is sometimes consumed by microorganisms,[23] but it is a significant groundwater contaminant due to its high solubility in water. The LD50 of acetone for fish is 8.3 g/l of water (or about 0.8%) over 96 hours, and its environmental half-life is about 1 to 10 days. Acetone may pose a significant risk of oxygen depletion in aquatic systems due to the microbial activity consuming it.[24]
Acetone peroxide
Main article: acetone peroxideWhen oxidized, acetone forms acetone peroxide as a byproduct, which is a highly unstable compound. It may be formed accidentally, e.g. when waste hydrogen peroxide is poured into waste solvent containing acetone. Acetone peroxide is more than ten times as sensitive to friction and shock as nitroglycerin[citation needed]. Due to its instability, it is rarely used, despite its easy chemical synthesis.
References
- ^ Merck Index, 11th Edition, 58
- ^ "Acetone – PubChem Public Chemical Database". The PubChem Project. USA: National Center for Biotechnology Information. http://pubchem.ncbi.nlm.nih.gov/summary/summary.cgi?cid=180.
- ^ a b c "Acetone". NIST Chemistry WebBook. USA: National Institute of Standards and Technology. http://webbook.nist.gov/cgi/cbook.cgi?ID=67-64-1.
- ^ Klamt, Andreas (2005). COSMO-RS: From Quantum Chemistry to Fluid Phase Thermodynamics and Drug Design. Elsevier. pp. 92–94. ISBN 0444519947, 9780444519948.
- ^ Ash, Michael; Ash, Irene (2004). Handbook of preservatives. Synapse Information Resources, Inc.. p. 369. ISBN 1890595667.
- ^ "Acetone CHROMASOLV® Plus, for HPLC, ≥99.9%". Sigma-Aldrich. http://www.sigmaaldrich.com/catalog/ProductDetail.do?lang=en&N4=650501%7CSIAL&N5=SEARCH_CONCAT_PNO. Retrieved 15 September 2011.
- ^ a b c Acetone, World Petrochemicals report, January 2010
- ^ a b c d e f Stylianos Sifniades, Alan B. Levy, “Acetone” in Ullmann’s Encyclopedia of Industrial Chemistry, Wiley-VCH, Weinheim, 2005.
- ^ a b c EO Camara Greiner and C Funada (june 2010). "CEH Marketing Research Report: ACETONE". Chemical Economics Handbook. SRI consulting. http://www.sriconsulting.com/CEH/Private/Reports/604.5000//. Retrieved March 2011.
- ^ "Acetone Uses and Market Data". ICIS.com. October 2010. http://www.icis.com/v2/chemicals/9074858/acetone/uses.html. Retrieved 2011-03-21.
- ^ NALGENE Labware – Technical Data
- ^ Weiner, Myra L.; Lois A. Kotkoskie (1999). Excipient Toxicity and Safety. pp. 32. ISBN 0824782100, 9780824782108.
- ^ Inactive Ingredient Search for Approved Drug Products, FDA/Center for Drug Evaluation and Research
- ^ Mine Safety and Health Administration (MSHA) – Safety Hazard Information – Special Hazards of Acetylene
- ^ History – Acetylene dissolved in acetone
- ^ What is an Azeotrope?
- ^ A. Lozano, B. Yip and R. K. Hanson (1992). "Acetone: a tracer for concentration measurements in gaseous flows by planar laser-induced fluorescence". Exp. Fluids 13 (6): 369–376. doi:10.1007/BF00223244.
- ^ a b Acetone MSDS
- ^ Basic Information on Acetone
- ^ Canadian Centre for Occupational Health and Safety. "Health Effects of Acetone". http://ccohs.ca/oshanswers/chemicals/chem_profiles/acetone/health_ace.html. Retrieved 2008-10-21.
- ^ Safety (MSDS) data for propanone
- ^ a b Likhodii SS, Serbanescu I, Cortez MA, Murphy P, Snead OC 3rd, Burnham WM (2003). "Anticonvulsant properties of acetone, a brain ketone elevated by the ketogenic diet". Ann Neurol. 54 (2): 219–226. doi:10.1002/ana.10634. PMID 12891674.
- ^ Acetone, Agency for Toxic Substances and Disease Registry ToxFAQs, 1995
- ^ Safety Data Sheet Acetone
External links
- International Chemical Safety Card 0087
- National Pollutant Inventory: Acetone
- NIOSH Pocket Guide to Chemical Hazards
- Hazardous substances databank entry at the national library of medicine
- SIDS Initial Assessment Report for Acetone from the Organisation for Economic Co-operation and Development (OECD)
- Calculation of vapor pressure, liquid density, dynamic liquid viscosity, surface tension of acetone
- Fisher Chemical MSDS
Cholesterol and steroid metabolic intermediates Mevalonate pathway to HMG-CoAto DMAPPGeranyl-Prephytoene diphosphate · PhytoeneNon-mevalonate pathway To Cholesterol Farnesyl pyrophosphate · Squalene · 2,3-Oxidosqualene · Lanosterol
Lanosterol · Lathosterol · 7-Dehydrocholesterol · Cholesterol
Lanosterol · Zymosterol · 7-Dehydrodesmosterol · Desmosterol · CholesterolSteroid Corticosteroids
(C21 pregnane)Androgens
(C19 androstane)Estrogens
(C18 estrane)Nonhuman biochemical families: prot · nucl · carb (glpr, alco, glys) · lipd (fata/i, phld, strd, gllp, eico) · amac/i · ncbs/i · ttpy/iCategories:- Household chemicals
- Cosmetics chemicals
- Biotechnology products
- Ketones
- Ketone solvents
- Fuel additives
- Excipients
Wikimedia Foundation. 2010.