- Mevalonic acid
-
Mevalonic acid 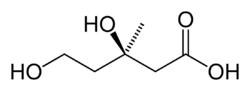
 (3R)-3,5-Dihydroxy-3-methylpentanoic acid
(3R)-3,5-Dihydroxy-3-methylpentanoic acidIdentifiers CAS number 150-97-0 Jmol-3D images Image 1 - C[C@@](O)(CCO)CC(=O)O
Properties Molecular formula C6H12O4 Molar mass 148.16 g mol−1  acid (verify) (what is:
acid (verify) (what is:  /
/ ?)
?)
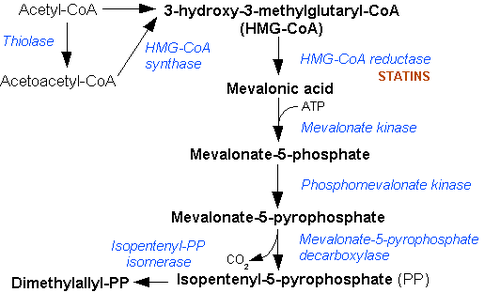
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references Mevalonic acid (MVA) is a key organic compound in biochemistry. The anion of mevalonic acid, the predominant form in biological media, is known as mevalonate. This compound is of major pharmaceutical importance. Drugs, such as the statins, stop the production of mevalonate by inhibiting HMG-CoA reductase.[1]
Chemistry
Mevalonic acid is very soluble in water and in polar organic solvents. It exists in equilibrium with the lactone, called mevalonolactone, formed by internal condensation of its terminal alcohol and carboxylic acid functional groups.
Biology
Mevalonic acid is a precursor in the biosynthetic pathway, known as the mevalonate pathway, that produces terpenes and steroids. Mevalonic acid is the primary precursor of isopentenyl pyrophosphate (IPP), that is in turn the basis for all terpenoids. Mevalonic acid is chiral and the (3R)-enantiomer is the only one that is biologically active.
References
Cholesterol and steroid metabolic intermediates Mevalonate pathway to HMG-CoAto DMAPPMevalonic acid · Phosphomevalonic acid · 5-Diphosphomevalonic acid · Isopentenyl pyrophosphate · Dimethylallyl pyrophosphateGeranyl-Prephytoene diphosphate · PhytoeneNon-mevalonate pathway To Cholesterol Farnesyl pyrophosphate · Squalene · 2,3-Oxidosqualene · Lanosterol
Lanosterol · Lathosterol · 7-Dehydrocholesterol · Cholesterol
Lanosterol · Zymosterol · 7-Dehydrodesmosterol · Desmosterol · CholesterolSteroid Corticosteroids
(C21 pregnane)Androgens
(C19 androstane)Estrogens
(C18 estrane)Nonhuman biochemical families: prot · nucl · carb (glpr, alco, glys) · lipd (fata/i, phld, strd, gllp, eico) · amac/i · ncbs/i · ttpy/iCategories:- Hydroxy acids
- Diols
Wikimedia Foundation. 2010.