- Estradiol
-
Not to be confused with 17α-estradiol.
Estradiol 
Systematic (IUPAC) name (17β)-estra-1,3,5(10)-triene-3,17-diol Clinical data Trade names Climara, Menostar AHFS/Drugs.com monograph Pregnancy cat. X (USA) Legal status S4 (Au), POM (UK), ℞-only (U.S.) Routes Oral, transdermal Pharmacokinetic data Bioavailability 97–99% is bound Metabolism Liver Half-life ~ 13 h Excretion Urine Identifiers CAS number 50-28-2 
57-91-0 (±)ATC code G03CA03 PubChem CID 5757 IUPHAR ligand 1012 DrugBank DB00783 ChemSpider 5554 
UNII 4TI98Z838E 
KEGG D00105 
ChEBI CHEBI:23965 
ChEMBL CHEMBL135 
Synonyms (8R,9S,13S,14S,17S)-13-methyl-6,7,8,9,11,12,14,15,16,17-decahydrocyclopenta[a]phenanthrene-3,17-diol Chemical data Formula C18H24O2 Mol. mass 272.38 SMILES eMolecules & PubChem  (what is this?) (verify)
(what is this?) (verify)Estradiol (E2 or 17β-estradiol, also oestradiol) is a sex hormone. Estradiol is abbreviated E2 as it has 2 hydroxyl groups in its molecular structure. Estrone has 1 (E1) and estriol has 3 (E3). Estradiol is about 10 times as potent as estrone and about 80 times as potent as estriol in its estrogenic effect. Except during the early follicular phase of the menstrual cycle, its serum levels are somewhat higher than that of estrone during the reproductive years of the human female. Thus it is the predominant estrogen during reproductive years both in terms of absolute serum levels as well as in terms of estrogenic activity. During menopause, estrone is the predominant circulating estrogen and during pregnancy estriol is the predominant circulating estrogen in terms of serum levels. Estradiol is also present in males, being produced as an active metabolic product of testosterone. The serum levels of estradiol in males (14[1] -55[1] pg/mL) are roughly comparable to those of postmenopausal women (< 35[1] pg/mL). Estradiol "in vivo" is interconvertible with estrone; estradiol to estrone conversion being favored. Estradiol has not only a critical impact on reproductive and sexual functioning, but also affects other organs, including the bones.
Contents
Synthesis
Estradiol, like other steroids, is derived from cholesterol. After side chain cleavage and using the delta-5 or the delta-4 pathway, androstenedione is the key intermediary. A fraction of the androstenedione is converted to testosterone, which in turn undergoes conversion to estradiol by an enzyme called aromatase. In an alternative pathway, androstenedione is aromatized to estrone, which is subsequently converted to estradiol.
Production
During the reproductive years, most estradiol in women is produced by the granulosa cells of the ovaries by the aromatization of androstenedione (produced in the theca folliculi cells) to estrone, followed by conversion of estrone to estradiol by 17β-hydroxysteroid dehydrogenase. Smaller amounts of estradiol are also produced by the adrenal cortex, and (in men), by the testes.
Estradiol is not produced in the gonads only: In both sexes, testosterone is converted by aromatization to estradiol. In particular, fat cells are active to convert precursors to estradiol, and will continue to do so even after menopause. Estradiol is also produced in the brain and in arterial walls.
Mechanism of action
Estradiol enters cells freely and interacts with a cytoplasmic target cell receptor. After the estrogen receptor has bound its ligand, estradiol can enter the nucleus of the target cell, and regulate gene transcription, which leads to formation of messenger RNA. The mRNA interacts with ribosomes to produce specific proteins that express the effect of estradiol upon the target cell.
Estradiol binds well to both estrogen receptors, ERα, and ERβ, in contrast to certain other estrogens, notably medications that preferentially act on one of these receptors. These medications are called selective estrogen receptor modulators, or SERMs.
Estradiol is the most potent naturally occurring estrogen.
Metabolism
In plasma, estradiol is largely bound to sex hormone-binding globulin, also to albumin. Only a fraction of 2.21% (± 0.04%) is free and biologically active, the percentage remaining constant throughout the menstrual cycle.[2] Deactivation includes conversion to less-active estrogens, such as estrone and estriol. Estriol is the major urinary metabolite. Estradiol is conjugated in the liver by sulfate and glucuronide formation and, as such, excreted via the kidneys. Some of the water-soluble conjugates are excreted via the bile duct, and partly reabsorbed after hydrolysis from the intestinal tract. This enterohepatic circulation contributes to maintaining estradiol levels.
Measurement
Serum estradiol measurement in women reflects primarily the activity of the ovaries. As such, they are useful in the detection of baseline estrogen in women with amenorrhea or menstrual dysfunction, and to detect the state of hypoestrogenicity and menopause. Furthermore, estrogen monitoring during fertility therapy assesses follicular growth and is useful in monitoring the treatment. Estrogen-producing tumors will demonstrate persistent high levels of estradiol and other estrogens. In precocious puberty, estradiol levels are inappropriately increased.
Ranges
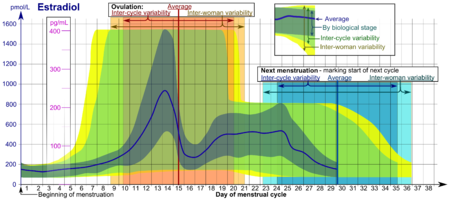 Reference ranges for the blood content of estradiol during the menstrual cycle[3]
Reference ranges for the blood content of estradiol during the menstrual cycle[3]
- The ranges denoted By biological stage may be used in closely monitored menstrual cycles in regard to other markers of its biological progression, with the time scale being compressed or stretched to how much faster or slower, respectively, the cycle progresses compared to an average cycle.
- The ranges denoted Inter-cycle variability are more appropriate to use in unmonitored cycles with only the beginning of menstruation known, but where the woman accurately knows her average cycle lengths and time of ovulation, and that they are somewhat averagely regular, with the time scale being compressed or stretched to how much a woman's average cycle length is shorter or longer, respectively, than the average of the population.
- The ranges denoted Inter-woman variability are more appropriate to use when the average cycle lengths and time of ovulation are unknown, but only the beginning of menstruation is given.Reference ranges for serum estradiol Patient type Lower limit Upper limit Unit Adult male 50[4] 200[4] pmol/L 14[1] 55[1] pg/mL Adult female (follicular
phase, day 5)70[4]
95% PI (standard)500[4]
95% PIpmol/L 110[5]
90% PI (used
in diagram)220[5]
90% PI19[1] (95% PI) 140[1] (95% PI) pg/mL 30[1] (90% PI) 60[1] (90% PI) Adult female (preovulatory
peak)400[4] 1500[4] pmol/L 110[1] 410[1] pg/mL Adult female
(luteal phase)70[4] 600[4] pmol/L 19[1] 160[1] pg/mL Adult female - free
(not protein bound)0.5[6] 9[6] pg/mL 1.7[6] 33[6] pmol/L Post-menopausal female N/A[4] < 130[4] pmol/L N/A[1] < 35[1] pg/mL In the normal menstrual cycle, estradiol levels measure typically <50 pg/ml at menstruation, rise with follicular development (peak: 200 pg/ml), drop briefly at ovulation, and rise again during the luteal phase for a second peak. At the end of the luteal phase, estradiol levels drop to their menstrual levels unless there is a pregnancy.
During pregnancy, estrogen levels, including estradiol, rise steadily toward term. The source of these estrogens is the placenta, which aromatizes prohormones produced in the fetal adrenal gland.
Effects
Female reproduction
In the female, estradiol acts as a growth hormone for tissue of the reproductive organs, supporting the lining of the vagina, the cervical glands, the endometrium, and the lining of the fallopian tubes. It enhances growth of the myometrium. Estradiol appears necessary to maintain oocytes in the ovary. During the menstrual cycle, estradiol produced by the growing follicle triggers, via a positive feedback system, the hypothalamic-pituitary events that lead to the luteinizing hormone surge, inducing ovulation. In the luteal phase, estradiol, in conjunction with progesterone, prepares the endometrium for implantation. During pregnancy, estradiol increases due to placental production. In baboons, blocking of estrogen production leads to pregnancy loss, suggesting estradiol has a role in the maintenance of pregnancy. Research is investigating the role of estrogens in the process of initiation of labor. Actions of estradiol are required before prior exposure of progesterone in the luteal phase.
Sexual development
The development of secondary sex characteristics in women is driven by estrogens, to be specific, estradiol. These changes are initiated at the time of puberty, most are enhanced during the reproductive years, and become less pronounced with declining estradiol support after the menopause. Thus, estradiol enhances breast development, and is responsible for changes in the body shape, affecting bones, joints and fat deposition. Fat structure and skin composition are modified by estradiol.
Male reproduction
The effect of estradiol (and estrogens) upon male reproduction is complex. Estradiol is produced by action of aromatase mainly in the Leydig cells of the mammalian testis, but also by some germ cells and the Sertoli cells of immature mammals [7]. It functions to prevent apoptosis of male sperm cells.[8]
Several studies have noted sperm counts have been declining in many parts of the world, and estrogen exposure in the environment has been postulated to be the cause.[9] Suppression of estradiol production in a subpopulation of subfertile men may improve the semen analysis.[10]
Males with sex chromosome genetic conditions, such as Klinefelters syndrome, will have a higher level of estradiol.
Bone
Estradiol has a profound effect on bone. Individuals without it (or other estrogens) will become tall and eunuchoid, as epiphyseal closure is delayed or may not take place. Bone structure is affected also, resulting in early osteopenia and osteoporosis.[11] Also, women past menopause experience an accelerated loss of bone mass due to a relative estrogen deficiency.
Liver
Estradiol has complex effects on the liver. It can lead to cholestasis. It affects the production of multiple proteins, including lipoproteins, binding proteins, and proteins responsible for blood clotting.
Brain
Estrogens can be produced in the brain from steroid precursors. As antioxidants, they have been found to have neuroprotective function.[12]
The positive and negative feedback loops of the menstrual cycle involve ovarian estradiol as the link to the hypothalamic-pituitary system to regulate gonadotropins.
Estrogen is considered to play a significant role in women’s mental health, with links suggested between the hormone level, mood and well-being. Sudden drops or fluctuations in, or long periods of sustained low levels of estrogen may be correlated with significant mood-lowering. Clinical recovery from depression postpartum, perimenopause, and postmenopause was shown to be effective after levels of estrogen were stabilized and/or restored.[13][14]
Blood vessels
Estrogen affects certain blood vessels. Improvement in arterial blood flow has been demonstrated in coronary arteries.[15]
Oncogene
Estrogen is suspected to activate certain oncogenes, as it supports certain cancers, notably breast cancer and cancer of the uterine lining. In addition, several benign gynecologic conditions are dependent on estrogen, such as endometriosis, leiomyomata uteri, and uterine bleeding.
Pregnancy
The effect of estradiol, together with estrone and estriol, in pregnancy is less clear. They may promote uterine blood flow, myometrial growth, stimulate breast growth and at term, promote cervical softening and expression of myometrial oxytocin receptors.
Role in sex differentiation of the brain
One of the fascinating twists to mammalian sex differentiation is that estradiol is one of the two active metabolites of testosterone in males (the other being dihydrotestosterone), and since fetuses of both sexes are exposed to similarly high levels of maternal estradiol, this source cannot have a significant impact on prenatal sex differentiation. Estradiol cannot be transferred readily from the circulation into the brain, whereas testosterone can; thus sex differentiation can be caused by the testosterone in the brain of most male mammals, including humans, aromatizing in significant amounts into estradiol. There is also now evidence the programming of adult male sexual behavior in animals is largely dependent on estradiol produced in the central nervous system during prenatal life and early infancy from testosterone.[16] However, it is not yet known whether this process plays a minimal or significant part in human sexual behaviors although evidence from other mammals tends to indicate that it does.[17]
Recently, the volumes of sexually dimorphic brain structures in phenotypical males were found to change to approximate those of typical female brain structures when exposed to estradiol over a period of months.[18] This would suggest estradiol has a significant part to play in sex differentiation of the brain, both prenatally and throughout life.
Estradiol medication
Estrogen is marketed in a number of ways to address issues of hypoestrogenism. Thus, there are oral, transdermal, topical, injectable, and vaginal preparations. Furthermore, the estradiol molecule may be linked to an alkyl group at C3 position to facilitate the administration. Such modifications give rise to estradiol acetate (oral and vaginal applications) and to estradiol cypionate (injectable).
Oral preparations are not necessarily predictably absorbed, and are subject to a first pass through the liver, where they can be metabolized, and also initiate unwanted side effects. Therefore, alternative routes of administration that bypass the liver before primary target organs are hit have been developed. Transdermal and transvaginal routes are not subject to the initial liver passage.
Ethinylestradiol, the most common estrogen ingredient in combined oral contraceptive pills, is a more profound alteration of the estradiol structure.
Therapy
 Nolvadex (tamoxifen) 20 mg
Nolvadex (tamoxifen) 20 mg
 Arimidex (anastrozole) 1 mg
Arimidex (anastrozole) 1 mg See also: Estrogen patch
See also: Estrogen patchBlocking estrogens
Inducing a state of hypoestrogenism may be beneficial in certain situations where estrogens are contributing to unwanted effects, e.g., certain forms of breast cancer, gynecomastia, transgenderism, and premature closure of epiphyses. Estrogen levels can be reduced by inhibiting production using gonadotropin-releasing factor agonists (GnRH agonists) or blocking the aromatase enzyme using an aromatase inhibitor, such as anastrozole, or with an estrogen receptor antagonist, such as tamoxifen. Flaxseed is known to reduce estradiol.[19]
Hormonal contraception
A derivative form of estradiol, ethinylestradiol, is a major component of hormonal contraceptive devices. Combined forms of hormonal contraception contain ethinylestradiol and a progestin, which both contribute to the inhibition of GnRH, LH, and FSH, which accounts for the ability of these birth control methods to prevent ovulation and thus prevent pregnancy. Other types of hormonal birth control contain only progestins and no ethinylestradiol.
List of estradiol medications
Products
- Oral versions: estradiol acetate (Estrace), estradiol valerate (Estrofem)
- Transdermal preparation: Alora, Climara, Vivelle-Dot, Menostar, Estraderm TTS, Estraderm MX, EvaMist
- Ointments: Divigel, Estrasorb Topical, Estrogel, Elestrin
- Injection: estradiol cypionate, estradiol valerate
- Vaginal ointment: Estrace Vaginal Cream
- Vaginal ring: Estring (estradiol acetate), Femring
- Estradiol combined with a progestin: Activella, AngeliQ, Cyclo-Progynova
Hormone replacement therapy
If severe side effects of low levels of estradiol in a woman's blood are experienced (commonly at the beginning of menopause or after oophorectomy), hormone replacement therapy may be prescribed. Often such therapy is combined with a progestin.
Estrogen therapy may be used in treatment of infertility in women when there is a need to develop sperm-friendly cervical mucus or an appropriate uterine lining. This is often prescribed in combination with clomifene.
Estrogen therapy can also be used to treat advanced prostate cancer, as well as to relieve symptoms of breast cancer.[20][21]
Estrogen therapy is also used to maintain female hormone levels in male-to-female transsexual women.
Notes
Not all products are available worldwide. Estradiol is also part of conjugated estrogen preparations, such as Premarin, though it is not the major ingredient (Premarin consists of hundreds of estrogen derivatives. As the name indicates, it comes from pregnant mares' urine.)
Adverse effects
Adverse effects, which may occur as a result of use of estradiol and have been associated with estrogen and/or progestin therapy, include changes in vaginal bleeding, dysmenorrhea, increase in size of uterine leiomyomata, vaginitis including vaginal candidiasis, changes in cervical secretion and cervical ectropion, ovarian cancer, endometrial hyperplasia, endometrial cancer, nipple discharge, galactorrhea, fibrocystic breast changes and breast cancer. Cardiovascular effects include chest pain, deep and superficial venous thrombosis, pulmonary embolism, thrombophlebitis, myocardial infarction, stroke, and increased blood pressure. Gastrointestinal effects include nausea and vomiting, abdominal cramps, bloating, diarrhea, dyspepsia, dysuria, gastritis, cholestatic jaundice, increased incidence of gallbladder disease, pancreatitis, or enlargement of hepatic hemangiomas. Skin adverse effects include chloasma or melasma that may continue despite discontinuation of the drug. Other effects on the skin include erythema multiforme, erythema nodosum, otitis media, hemorrhagic eruption, loss of scalp hair, hirsutism, pruritus, or rash. Adverse effects on the eyes include retinal vascular thrombosis, steepening of corneal curvature or intolerance to contact lenses. Adverse central nervous system effects include headache, migraine, dizziness, mental depression, chorea, nervousness/anxiety, mood disturbances, irritability, and worsening of epilepsy. Other adverse effects include changes in weight, reduced carbohydrate tolerance, worsening of porphyria, edema, arthralgias, bronchitis, leg cramps, hemorrhoids, changes in libido, urticaria, angioedema, anaphylactic reactions, syncope, toothache, tooth disorder, urinary incontinence, hypocalcemia, exacerbation of asthma, and increased triglycerides.[22][23]
Estrogen combined with medroxyprogesterone is associated with an increased risk of dementia. It is not known whether estradiol taken alone is associated with an increased risk of dementia. Estrogens should only be used for the shortest possible time and at the lowest effective dose due to these risks. Attempts to gradually reduce the medication via a dose taper should be made every three to six months.[22]
Interactions
St John's wort, phenobarbital, carbamazepine and rifampin decrease the levels of estrogens, such as estradiol, by speeding up its metabolism, whereas erythromycin, clarithromycin, ketoconazole, itraconazole, ritonavir and grapefruit juice may slow down metabolism, leading to increased levels in the blood plasma.[22]
Contraindications
Estradiol should be avoided when there is undiagnosed abnormal genital bleeding, known, suspected or a history of breast cancer, current treatment for metastatic disease, known or suspected estrogen-dependent neoplasia, deep vein thrombosis, pulmonary embolism or history of these conditions, active or recent arterial thromboembolic disease such as stroke, myocardial infarction, liver dysfunction or disease. Estradiol should not be taken by people with a hypersensitivity/allergy or those who are pregnant or are suspected pregnancy.[22]
See also
- Estrogen insensitivity syndrome
- Gender
- Androgen
- Oral contraceptive formulations
- Phytoestrogens, the family of plant chemicals which can act on estradiol receptive tissue in mammals, although the exact mechanism at hand is unclear.
References
- ^ a b c d e f g h i j k l m n o Derived from molar values using molar mass of 272.38g/mol
- ^ Wu CH, Motohashi T, Abdel-Rahman HA, Flickinger GL, Mikhail G (August 1976). "Free and protein-bound plasma estradiol-17 beta during the menstrual cycle". J. Clin. Endocrinol. Metab. 43 (2): 436–45. doi:10.1210/jcem-43-2-436. PMID 950372.
- ^ References and further description of values are given in image page in Wikimedia Commons at Commons:File:Estradiol during menstrual cycle.png.
- ^ a b c d e f g h i j GPNotebook — reference range (oestradiol) Retrieved on September 27, 2009
- ^ a b Values taken from day 1 after LH surge in: Stricker R, Eberhart R, Chevailler MC, Quinn FA, Bischof P, Stricker R (2006). "Establishment of detailed reference values for luteinizing hormone, follicle stimulating hormone, estradiol, and progesterone during different phases of the menstrual cycle on the Abbott ARCHITECT analyzer". Clin. Chem. Lab. Med. 44 (7): 883–7. doi:10.1515/CCLM.2006.160. PMID 16776638. http://www.reference-global.com/doi/abs/10.1515/CCLM.2006.160?url_ver=Z39.88-2003&rfr_id=ori:rid:crossref.org&rfr_dat=cr_pub%3dpubmed. as PDF
- ^ a b c d Total amount multiplied by 0.022 according to 2.2% presented in: Wu CH, Motohashi T, Abdel-Rahman HA, Flickinger GL, Mikhail G (August 1976). "Free and protein-bound plasma estradiol-17 beta during the menstrual cycle". J. Clin. Endocrinol. Metab. 43 (2): 436–45. doi:10.1210/jcem-43-2-436. PMID 950372.
- ^ Carreau, S; Lambard, S; Delalande, C; Denis-Galeraud, I; Bourguiba, S; Bourguiba, Sonia (2003). "Aromatase expression and role of estrogens in male gonad : a review". Reproductive Biology and Endocrinology 1: 35. doi:10.1186/1477-7827-1-35. PMC 155680. PMID 12747806. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=155680.
- ^ Pentikäinen, V; Erkkilä, K; Suomalainen, L; Parvinen, M; Dunkel, L (2000). "Estradiol acts as a germ cell survival factor in the human testis in vitro". The Journal of clinical endocrinology and metabolism 85 (5): 2057–67. doi:10.1210/jc.85.5.2057. PMID 10843196.
- ^ Sharpe, RM; Skakkebaek, NE (1993). "Are oestrogens involved in falling sperm counts and disorders of the male reproductive tract?". Lancet 341 (8857): 1392–5. doi:10.1016/0140-6736(93)90953-E. PMID 8098802.
- ^ Raman, JD; Schlegel, PN (2002). "Aromatase inhibitors for male infertility". The Journal of urology 167 (2 Pt 1): 624–9. doi:10.1016/S0022-5347(01)69099-2. PMID 11792932.
- ^ Carani, C; Qin, K; Simoni, M; Faustini-Fustini, M; Serpente, S; Boyd, J; Korach, KS; Simpson, ER (1997). "Effect of testosterone and estradiol in a man with aromatase deficiency". The New England journal of medicine 337 (2): 91–5. doi:10.1056/NEJM199707103370204. PMID 9211678.
- ^ Behl C, Widmann M, Trapp T, Holsboer F (November 1995). "17-beta estradiol protects neurons from oxidative stress-induced cell death in vitro". Biochem. Biophys. Res. Commun. 216 (2): 473–82. doi:10.1006/bbrc.1995.2647. PMID 7488136.
- ^ Douma SL, Husband C, O'Donnell ME, Barwin BN, Woodend AK (2005). "Estrogen-related mood disorders: reproductive life cycle factors". ANS Adv Nurs Sci 28 (4): 364–75. PMID 16292022.
- ^ Lasiuk GC, Hegadoren KM (October 2007). "The effects of estradiol on central serotonergic systems and its relationship to mood in women". Biol Res Nurs 9 (2): 147–60. doi:10.1177/1099800407305600. PMID 17909167.
- ^ Collins, P; Rosano, GM; Sarrel, PM; Ulrich, L; Adamopoulos, S; Beale, CM; McNeill, JG; Poole-Wilson, PA (1995). "17 beta-Estradiol attenuates acetylcholine-induced coronary arterial constriction in women but not men with coronary heart disease". Circulation 92 (1): 24–30. PMID 7788912.
- ^ Harding, Prof. Cheryl F. (June 2004). "Hormonal Modulation of Singing: Hormonal Modulation of the Songbird Brain and Singing Behavior". Ann. N.Y. Acad. Sci. (The New York Academy of Sciences) 1016: 524–539. doi:10.1196/annals.1298.030. PMID 15313793. http://www.annalsnyas.org/content/vol1016/issue1/index.dtl. Retrieved 2007-03-07.
- ^ Simerly, Richard B. (2002-03-27). "Wired for reproduction: organization and development of sexually dimorphic circuits in the mammalian forebrain" (pdf). Annual Rev. Neurosci. 25: 507–536. doi:10.1146/annurev.neuro.25.112701.142745. PMID 12052919. http://www.healthsystem.virginia.edu/internet/neuroscience/BehavioralNeuroscience/Simerley-EFR-1-4.pdf. Retrieved 2007-03-07.
- ^ Hulshoff, Cohen-Kettenis et al. (July 2006). "Changing your sex changes your brain: influences of testosterone and estrogen on adult human brain structure". European Journal of Endocrinology 155 (155): 107–114. doi:10.1530/eje.1.02248. http://www.eje-online.org/cgi/content/abstract/155/suppl_1/S107.
- ^ Chevallier, Andrew (2000). Gillian Emerson-Roberts. ed. Encyclopedia of Herbal Medicine: The Definitive Home Reference Guide to 550 Key Herbs with all their Uses as Remedies for Common Ailments. DK Publishing. ISBN 0-7894-6783-6.
- ^ Ockrim JL, Lalani el-N, Kakkar AK, Abel PD (August 2005). "Transdermal estradiol therapy for prostate cancer reduces thrombophilic activation and protects against thromboembolism". J. Urol. 174 (2): 527–33; discussion 532–3. doi:10.1097/01.ju.0000165567.99142.1f. PMID 16006886. http://linkinghub.elsevier.com/retrieve/pii/00005392-200508000-00036.
- ^ Carruba G, Pfeffer U, Fecarotta E, et al. (March 1994). "Estradiol inhibits growth of hormone-nonresponsive PC3 human prostate cancer cells". Cancer Res. 54 (5): 1190–3. PMID 8118804. http://cancerres.aacrjournals.org/cgi/pmidlookup?view=long&pmid=8118804.
- ^ a b c d Barr Laboratories, Inc. (March 2008). "ESTRACE TABLETS, (estradiol tablets, USP)" (PDF). wcrx.com. http://www.wcrx.com/pdfs/pi/pi_estrace_wc_imprint.pdf. Retrieved 27 January 2010.
- ^ Pfizer (August 2008). "ESTRING (estradiol vaginal ring)" (PDF). http://media.pfizer.com/files/products/uspi_estring.pdf.
Additional images
Endocrine system: hormones (Peptide hormones · Steroid hormones) Endocrine
glandsTestis: testosterone · AMH · inhibin
Ovary: estradiol · progesterone · activin and inhibin · relaxin (pregnancy)
Placenta: hCG · HPL · estrogen · progesteroneIslet-Acinar
AxisNon-end.
glandsThymus: Thymosin (Thymosin α1, Thymosin beta) · Thymopoietin · Thymulin
Digestive system: Stomach: gastrin · ghrelin · Duodenum: CCK · GIP · secretin · motilin · VIP · Ileum: enteroglucagon · peptide YY · Liver/other: Insulin-like growth factor (IGF-1, IGF-2)
Adipose tissue: leptin · adiponectin · resistin
Kidney: JGA (renin) · peritubular cells (EPO) · calcitriol · prostaglandin
Heart: Natriuretic peptide (ANP, BNP)Estrogens and progestogens (G03C-D, L02) Progestogens/
progestins
(progesterone)AgonistAndrostene (Drospirenone) • 19-norprogesterone (Nomegestrol • Promegestone • Trimegestone) • 19-nortestosterone (Dienogest)Other/
ungroupedPregnenedione (Gestonorone) • Pregnene (Ethisterone) • Pregnadiene (Medrogestone • Melengestrol) • Norpregnane (Norgestrienone) • Lynestrenol • Norethynodrel • Tibolone • Dydrogesterone • Quingestanolantagonist: MifepristoneAsoprisnil • CDB-4124 • Ulipristal acetateEstrogens AgonistDiosgenin • Estradiol (Ethinylestradiol#/Mestranol • Estradiol 17 beta-cypionate# • Polyestradiol phosphate) • Estrone (Estrone sulfate) • Estriol • Promestriene • Equilenin • EquilinAfimoxifene • Arzoxifene • Bazedoxifene • Cyclofenil • Lasofoxifene • Ormeloxifene • Raloxifene • Tamoxifen • Toremifenepure antagonist: FulvestrantCholesterol and steroid metabolic intermediates Mevalonate pathway to HMG-CoAto DMAPPGeranyl-Prephytoene diphosphate · PhytoeneNon-mevalonate pathway To Cholesterol Farnesyl pyrophosphate · Squalene · 2,3-Oxidosqualene · Lanosterol
Lanosterol · Lathosterol · 7-Dehydrocholesterol · Cholesterol
Lanosterol · Zymosterol · 7-Dehydrodesmosterol · Desmosterol · CholesterolSteroid Corticosteroids
(C21 pregnane)Androgens
(C19 androstane)Estrogens
(C18 estrane)Nonhuman biochemical families: prot · nucl · carb (glpr, alco, glys) · lipd (fata/i, phld, strd, gllp, eico) · amac/i · ncbs/i · ttpy/iCategories:- Estrogens
- Phenols
- Steroids
- Alcohols
- Diols
Wikimedia Foundation. 2010.