- Medroxyprogesterone
-
This article is about a synthetic progestin ligand. For the clinically prescribed drug, see medroxyprogesterone 17-acetate.
Medroxyprogesterone 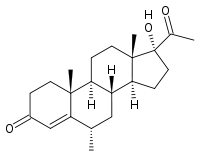 17-acetyl-17-hydroxy- 6,10,13-trimethyl- 1,2,6,7,8,9,10,11,12,13,14,15,16,17- tetradecahydrocyclopenta[a]phenanthren-3-one
17-acetyl-17-hydroxy- 6,10,13-trimethyl- 1,2,6,7,8,9,10,11,12,13,14,15,16,17- tetradecahydrocyclopenta[a]phenanthren-3-oneIdentifiers CAS number 520-85-4 
PubChem 10631 ChemSpider 10185 
UNII HSU1C9YRES 
DrugBank DB00603 KEGG D08166 
ChEBI CHEBI:6715 
ChEMBL CHEMBL279067 
Jmol-3D images Image 1 - O=C4\C=C2/[C@]([C@H]1CC[C@@]3([C@@](O)(C(=O)C)CC[C@H]3[C@@H]1C[C@@H]2C)C)(C)CC4
- InChI=1S/C22H32O3/c1-13-11-16-17(20(3)8-5-15(24)12-19(13)20)6-9-21(4)18(16)7-10-22(21,25)14(2)23/h12-13,16-18,25H,5-11H2,1-4H3/t13-,16+,17-,18-,20+,21-,22-/m0/s1

Key: FRQMUZJSZHZSGN-HBNHAYAOSA-N
InChI=1/C22H32O3/c1-13-11-16-17(20(3)8-5-15(24)12-19(13)20)6-9-21(4)18(16)7-10-22(21,25)14(2)23/h12-13,16-18,25H,5-11H2,1-4H3/t13-,16+,17-,18-,20+,21-,22-/m0/s1
Key: FRQMUZJSZHZSGN-HBNHAYAOBM
Properties Molecular formula C22H32O3 Molar mass 344.488 g/mol  (verify) (what is:
(verify) (what is:  /
/ ?)
?)
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references Medroxyprogesterone (MP) is a pregnane that acts as a progestin. An acylated derivative, medroxyprogesterone 17-acetate (MPA) is clinically used as a drug.[1] Compared to MPA, MP is approximately 100 fold less potent as a progestin.[2] MP is also a metabolite of MPA.[3]
While medroxyprogesterone is sometimes used as a synonym for medroxyprogesterone 17-acetate,[1] what is normally being administered is medroxyprogesterone 17-acetate and not medroxyprogesterone.[4]
Medroxyprogesterone is used to regulate irregular periods in a woman's menstral cycle. This drug is also used with women going through menopause.
See also
References
- ^ a b "MedroxyPROGESTERone: Drug Information Provided by Lexi-Comp". Merck Manual. 2009-12-01. http://www.merck.com/mmpe/lexicomp/medroxyprogesterone.html. Retrieved 2010-07-08.
- ^ Pullen MA, Laping N, Edwards R, Bray J (September 2006). "Determination of conformational changes in the progesterone receptor using ELISA-like assays". Steroids 71 (9): 792–8. doi:10.1016/j.steroids.2006.05.009. PMID 16784762.
- ^ Ishihara M, Kirdani Y, Osawa Y, Sandberg AA (January 1976). "The metabolic fate of medroxyprogesterone acetate in the baboon". J. Steroid Biochem. 7 (1): 65–70. doi:10.1016/0022-4731(76)90167-9. PMID 1271819.
- ^ Lenco W, Mcknight M, Macdonald AS (January 1975). "Effects of cortisone acetate, methylprednisolone and medroxyprogesterone on wound contracture and epithelization in rabbits". Ann. Surg. 181 (1): 67–73. doi:10.1097/00000658-197501000-00015. PMC 1343717. PMID 1119869. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=1343717.
Estrogens and progestogens (G03C-D, L02) Progestogens/
progestins
(progesterone)AgonistAndrostene (Drospirenone) • 19-norprogesterone (Nomegestrol • Promegestone • Trimegestone) • 19-nortestosterone (Dienogest)Other/
ungroupedPregnenedione (Gestonorone) • Pregnene (Ethisterone) • Pregnadiene (Medrogestone • Melengestrol) • Norpregnane (Norgestrienone) • Lynestrenol • Norethynodrel • Tibolone • Dydrogesterone • Quingestanolantagonist: MifepristoneEstrogens AgonistDiosgenin • Estradiol (Ethinylestradiol#/Mestranol • Estradiol 17 beta-cypionate# • Polyestradiol phosphate) • Estrone (Estrone sulfate) • Estriol • Promestriene • Equilenin • EquilinAfimoxifene • Arzoxifene • Bazedoxifene • Cyclofenil • Lasofoxifene • Ormeloxifene • Raloxifene • Tamoxifen • ToremifeneCategories:- Progestagens
Wikimedia Foundation. 2010.
