- Mestranol
-
Mestranol 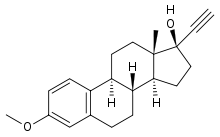
Systematic (IUPAC) name 3-Methoxy-19-nor-17α-pregna-1,3,5(10)-trien-20-yn-17-ol Clinical data AHFS/Drugs.com International Drug Names MedlinePlus a601050 Pregnancy cat. ? Legal status ? Identifiers CAS number 72-33-3 
ATC code None PubChem CID 6291 DrugBank DB01357 ChemSpider 6054 
UNII B2V233XGE7 
KEGG D00575 
ChEBI CHEBI:6784 
ChEMBL CHEMBL1201151 
Synonyms (8S,9S,13S,14S,17S)-17-ethynyl-3-methoxy-13-methyl-7,8,9,11,12,14,15,16-octahydro-6H-cyclopenta[a]phenanthren-17-ol Chemical data Formula C21H26O2 Mol. mass 310.43 g/mol SMILES eMolecules & PubChem  (what is this?) (verify)
(what is this?) (verify)Mestranol is the 3-methyl ether of ethinylestradiol. It was the estrogen used in many of the first oral contraceptives.
It is a biologically inactive prodrug of ethinylestradiol to which it is demethylated in the liver with a conversion efficiency of 70% (50 µg of mestranol is pharmacokinetically bioequivalent to 35 µg of ethinylestradiol).[1]
References
Estrogens and progestogens (G03C-D, L02) Progestogens/
progestins
(progesterone)AgonistAndrostene (Drospirenone) • 19-norprogesterone (Nomegestrol • Promegestone • Trimegestone) • 19-nortestosterone (Dienogest)Other/
ungroupedPregnenedione (Gestonorone) • Pregnene (Ethisterone) • Pregnadiene (Medrogestone • Melengestrol) • Norpregnane (Norgestrienone) • Lynestrenol • Norethynodrel • Tibolone • Dydrogesterone • Quingestanolantagonist: MifepristoneEstrogens AgonistDiosgenin • Estradiol (Ethinylestradiol#/Mestranol • Estradiol 17 beta-cypionate# • Polyestradiol phosphate) • Estrone (Estrone sulfate) • Estriol • Promestriene • Equilenin • EquilinAfimoxifene • Arzoxifene • Bazedoxifene • Cyclofenil • Lasofoxifene • Ormeloxifene • Raloxifene • Tamoxifen • Toremifene#WHO-EM. ‡Withdrawn from market. Clinical trials: †Phase III. §Never to phase III 
This drug article relating to the genito-urinary system is a stub. You can help Wikipedia by expanding it.
