- Megestrol
-
Megestrol 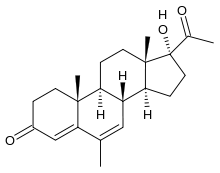
Systematic (IUPAC) name 17-acetyl-17-hydroxy-6,10, 13-trimethyl-2,8,9,11,12,14,15,16- octahydro-1H-cyclopenta[ a] phenanthren-3-one Clinical data Trade names Megace AHFS/Drugs.com monograph MedlinePlus a682003 Pregnancy cat. contraindicated Legal status ? Pharmacokinetic data Half-life 34 hours Identifiers CAS number 3562-63-8 
ATC code G03AC05 G03DB02, L02AB01 PubChem CID 19090 DrugBank APRD01092 ChemSpider 18023 
UNII EA6LD1M70M 
KEGG D08167 
ChEMBL CHEMBL1201139 
Chemical data Formula C22H30O3 Mol. mass 342.472 g/mol SMILES eMolecules & PubChem  (what is this?) (verify)
(what is this?) (verify)Megestrol is a progesterone derivative with antineoplastic properties used in the treatment of advanced carcinoma of the breast and endometrium. When given in relatively high doses, Megestrol can substantially increase appetite in most individuals, even those with advanced cancer. It is also used to boost appetite in individuals with other cancers or HIV/AIDS.
Megestrol acetate oral suspension (a form of Megestrol) is used primarily as an appetite enhancer. The method of appetite enhancement is not known, but it can cause high blood sugar.[1]
Currently, it is manufactured under the trade name Megace.[2]
Little is known about the drug's chemical interactions. Doses range from 40 mg three times a day, 30 minutes before meals, to 800 mg once a day. Side effects may include diarrhea, rash, and impotence. Males may have some feminizing effects such as gynecomastia (breast development). It is a category X U.S. Food and Drug Administration drug, which means cannot be used in pregnancy as it crosses the placenta and malignantly affects the fetus.[3]. Natural alternatives to Megace although not quite as effective are found in natural herbs such as gentian, ginger, and others [4]
It may cause adrenal insufficiency.[5]
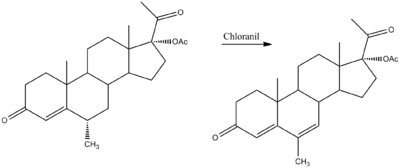
Synthesis
Medroxyprogesterone acetate is on the left. Megestrol acetate is on the right.[6]
Notes
- ^ "Megace ES: megestrol acetate 625 mg/5mL oral suspension | Side Effects with Megace ES". http://www.megacees.com/pat-isi.html. Retrieved 2011-06-28.
- ^ "Malnutrition and Cachexia Treatment | Megace ES (megestrol acetate)". http://www.megacees.com/. Retrieved 2009-05-17.
- ^ http://www.answers.com/topic/megestrol-megace
- ^ ""Megace and Megestrol Alternatives | EATMoR Appetite Stimulant". http://www.appetitestimulants.net/megace-megestrol.html. Retrieved 2011-10-19.
- ^ Bulchandani D, Nachnani J, Amin A, May J (August 2008). "Megestrol acetate-associated adrenal insufficiency". Am J Geriatr Pharmacother 6 (3): 167–72. doi:10.1016/j.amjopharm.2008.08.004. PMID 18775392. http://linkinghub.elsevier.com/retrieve/pii/S1543-5946(08)00043-3.
- ^ Ringold, H. J.; Ruelas, J. P.; Batres, E.; Djerassi, C. (1959). Journal of the American Chemical Society 81 (14): 3712. doi:10.1021/ja01523a055.
References
- Raney MS, Anding R, Fay V, Polk G (2000). "A pilot study to assess the use of megesterol acetate to promote weight gain in frail elderly persons residing in long-term care". J Am Med Dir Assoc 1 (4): 154–8. PMID 12816553.
- Deutsch J, Kolhouse JF (July 2004). "Assessment of gastrointestinal function and response to megesterol acetate in subjects with gastrointestinal cancers and weight loss". Support Care Cancer 12 (7): 503–10. doi:10.1007/s00520-004-0615-4. PMID 15064933.
Estrogens and progestogens (G03C-D, L02) Progestogens/
progestins
(progesterone)AgonistAndrostene (Drospirenone) • 19-norprogesterone (Nomegestrol • Promegestone • Trimegestone) • 19-nortestosterone (Dienogest)Other/
ungroupedPregnenedione (Gestonorone) • Pregnene (Ethisterone) • Pregnadiene (Medrogestone • Melengestrol) • Norpregnane (Norgestrienone) • Lynestrenol • Norethynodrel • Tibolone • Dydrogesterone • Quingestanolantagonist: MifepristoneAsoprisnil • CDB-4124 • Ulipristal acetateEstrogens AgonistDiosgenin • Estradiol (Ethinylestradiol#/Mestranol • Estradiol 17 beta-cypionate# • Polyestradiol phosphate) • Estrone (Estrone sulfate) • Estriol • Promestriene • Equilenin • EquilinAfimoxifene • Arzoxifene • Bazedoxifene • Cyclofenil • Lasofoxifene • Ormeloxifene • Raloxifene • Tamoxifen • Toremifenepure antagonist: Fulvestrant
This antineoplastic or immunomodulatory drug article is a stub. You can help Wikipedia by expanding it. This drug article relating to the genito-urinary system is a stub. You can help Wikipedia by expanding it.