- Ethinylestradiol
-
Ethinylestradiol 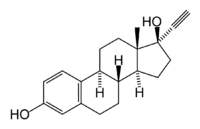
Systematic (IUPAC) name 19-Nor-17α-pregna-1,3,5(10)-trien-20-yne-3,17-diol Clinical data AHFS/Drugs.com International Drug Names MedlinePlus a604032 Pregnancy cat. X (USA) Legal status Rx-only (U.S.) Routes Oral, transdermal Pharmacokinetic data Bioavailability 97% is bound Metabolism Liver Half-life 36±13 hours Excretion Feces and Urine Identifiers CAS number 57-63-6 
ATC code G03CA01 L02AA03 PubChem CID 5991 DrugBank APRD00691 ChemSpider 5770 
UNII 423D2T571U 
KEGG D00554 
ChEBI CHEBI:4903 
ChEMBL CHEMBL1078384 
Chemical data Formula C20H24O2 Mol. mass 296.403 SMILES eMolecules & PubChem  (what is this?) (verify)
(what is this?) (verify)Ethynylestradiol (
 /ˌɛθɨnɨlˌiːstrəˈdaɪ.əl/), also ethynyl estradiol (EE), is a derivative of estradiol. Ethynyl estradiol is an orally bio-active estrogen used in almost all modern formulations of combined oral contraceptive pills. It is one of the most commonly used medications.
/ˌɛθɨnɨlˌiːstrəˈdaɪ.əl/), also ethynyl estradiol (EE), is a derivative of estradiol. Ethynyl estradiol is an orally bio-active estrogen used in almost all modern formulations of combined oral contraceptive pills. It is one of the most commonly used medications.The same contraindications and precautions apply for EE as with other estrogen medications.
Estinyl was a preparation of EE alone that was used for the management of menopausal symptoms and female hypogonadism.[1]
EE is released into the environment as a xenoestrogen from the urine and feces of people who take it as a medication.
Contents
History
The first orally active semisynthetic steroidal estrogen, ethinylestradiol (17α-ethynylestradiol), the 17α-ethynyl analog of estradiol, was synthesized in 1938 by Hans Herloff Inhoffen and Walter Hohlweg at Schering AG in Berlin.[2][3][4][5][6]
Ethinyl estradiol was approved by the FDA in the U.S. on June 25, 1943 and marketed by Schering as Estinyl.[7] The FDA withdrew approval of Estinyl effective June 4, 2004 at the request of Schering, who had discontinued marketing Estinyl.[8]
Pharmacology
While estradiol is readily absorbed when taken orally, it is also quickly inactivated by the liver. Substitution at C17 of the estrane steroid with an ethinyl group proved to provide an estrogen that is much more resistant to degradation and paved the way for the development of oral contraceptives.
EE is absorbed in the small intestine and reaches a serum peak about 2 hours later. It undergoes extensive metabolism in the liver involving the cytochrome P450 3A4 isoenzyme. EE and its metabolites are excreted with the bile. Due to the effect of enterohepatic circulation a second peak is seen several hours later. Individually, wide variations exist in the overall absorption process, and can be further modified by drugs (i.e. antibiotics) that affect the enterohepatic circulation or liver enzymes. In circulation EE is almost fully bound to plasma albumin. It is metabolized by hydroxylation of the aromatic ring and excreted in both feces and urine, in part as glucuronide and sulfate conjugate.
EE is hormonally effective by activating the estrogen receptor and thus is an estrogen. It finds its most common use in the estrogen-progestin combination preparations of oral contraceptives. Over time, formulations have decreased the EE dose from as high as 100 μg to as low as 10 μg in LoLoestrin Fe.[9]
Dosing
Depends on needs and age, see Consumer Information for more information.
Chemistry
Ethinylestradiol is prepared from estron in one step by ethinylation with ethine, sodium and sodium amide.[10]
See also
- Hormonal contraception
- Oral contraceptive formulations
- Diethylstilbestrol
- Esterified Estrogens
- Estradiol
- Estrone
- Estropipate
- Antineoplastic
- Osteoporosis prophylactic
- Ovarian hormone therapy
- Systemic Estrogen
- Birth Control
References
- ^ RxList.com - Estinyl (ethynyl estradiol)
- ^ Inhoffen, H. H.; Hohlweg, W. (1938). "Neue per os-wirksame weibliche Keimdrüsenhormon-Derivate: 17-Aethinyl-oestradiol und Pregnen-in-on-3-ol-17 (New female glandular derivatives active per os: 17α-ethynyl-estradiol and pregnen-in-on-3-ol-17)". Naturwissenschaften 26 (6): 96. doi:10.1007/BF01681040.
- ^ Maisel, Albert Q. (1965). The Hormone Quest. New York: Random House. OCLC 543168.
- ^ Petrow, Vladimir (December 1970). "The contraceptive progestagens". Chem Rev 70 (6): 713–26. doi:10.1021/cr60268a004. PMID 4098492.
- ^ Sneader, Walter (2005). "Hormone analogues". Drug discovery : a history. Hoboken, NJ: John Wiley & Sons. pp. 188–225. ISBN 0471899801.
- ^ Djerassi, Carl (January 2006). "Chemical birth of the pill". American Journal of Obstetrics and Gynecology 194 (1): 290–8. doi:10.1016/j.ajog.2005.06.010. PMID 16389046.
- ^ FDA (2007). "Drug details: Estinyl (ethinyl estradiol) NDA 005292". http://www.accessdata.fda.gov/scripts/cder/drugsatfda/index.cfm?fuseaction=Search.DrugDetails. search: Estinyl
- ^ FDA (May 5, 2004). "Schering Corp. et al.; Withdrawal of Approval of 92 New Drug Applications and 49 Abbreviated New Drug Applications. Notice". Federal Register 69 (87): 25124–30. http://edocket.access.gpo.gov/2004/pdf/04-10194.pdf.
- ^ "LoLoestrin Fe website". Warner Chilcott. http://www.loloestrin.com/index.jsp. Retrieved 15 October 2011.
- ^ Kleemann, Axel; Engel, Jürgen; Kutscher, Bernd; Reichert, Dieter (2009). Pharmaceutical Substances (5 ed.). Thieme-Verlag Stuttgart. ISBN 978-3-13-558405-8.
External links
- http://www.drugs.com/sfx/ethinyl-estradiol-side-effects.html
- http://www.drugs.com/cons/ethinyl-estradiol.html
Estrogens and progestogens (G03C-D, L02) Progestogens/
progestins
(progesterone)AgonistAndrostene (Drospirenone) • 19-norprogesterone (Nomegestrol • Promegestone • Trimegestone) • 19-nortestosterone (Dienogest)Other/
ungroupedPregnenedione (Gestonorone) • Pregnene (Ethisterone) • Pregnadiene (Medrogestone • Melengestrol) • Norpregnane (Norgestrienone) • Lynestrenol • Norethynodrel • Tibolone • Dydrogesterone • Quingestanolantagonist: MifepristoneAsoprisnil • CDB-4124 • Ulipristal acetateEstrogens AgonistAfimoxifene • Arzoxifene • Bazedoxifene • Cyclofenil • Lasofoxifene • Ormeloxifene • Raloxifene • Tamoxifen • Toremifenepure antagonist: FulvestrantCategories:- Alkynes
- Hormonal contraception
- Synthetic estrogens
- Diols
Wikimedia Foundation. 2010.

