- Cyclofenil
-
Not to be confused with Cycloguanil.
Cyclofenil 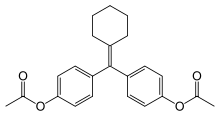
Systematic (IUPAC) name [4-[(4-acetoxyphenyl)-cyclohexylidene-methyl]phenyl] acetate Clinical data AHFS/Drugs.com International Drug Names Pregnancy cat. ? Legal status ? Routes Oral Identifiers CAS number 5189-40-2 ATC code G03GB01 PubChem CID 2898 ChemSpider 2795 UNII J468V64WZ1 
KEGG D01281 Synonyms bis(p-acetoxyphenyl)cyclohexylidenemethane Chemical data Formula C23H24O4 Mol. mass 364.434 g/mol SMILES eMolecules & PubChem Cyclofenil (INN, trade names Ondogyne, Sexovid, and others) is a selective estrogen receptor modulator.
It was investigated as a possible treatment for scleroderma in the 1980s, but was found to be ineffective.[1] Later study of its efficacy in treating Raynaud's phenomenon in people with scleroderma also found no statistically significant benefit.[2]
References
- ^ Torres MA, Furst DE (February 1990). "Treatment of generalized systemic sclerosis". Rheum Dis Clin North Am 16 (1): 217–41. PMID 2406809.
- ^ Pope J, Fenlon D, Thompson A, et al. (2000). Pope, Janet. ed. "Cyclofenil for Raynaud's phenomenon in progressive systemic sclerosis". Cochrane Database Syst Rev (2): CD000955. doi:10.1002/14651858.CD000955. PMID 10796397.
Estrogens and progestogens (G03C-D, L02) Progestogens/
progestins
(progesterone)AgonistAndrostene (Drospirenone) • 19-norprogesterone (Nomegestrol • Promegestone • Trimegestone) • 19-nortestosterone (Dienogest)Other/
ungroupedPregnenedione (Gestonorone) • Pregnene (Ethisterone) • Pregnadiene (Medrogestone • Melengestrol) • Norpregnane (Norgestrienone) • Lynestrenol • Norethynodrel • Tibolone • Dydrogesterone • Quingestanolantagonist: MifepristoneAsoprisnil • CDB-4124 • Ulipristal acetateEstrogens AgonistDiosgenin • Estradiol (Ethinylestradiol#/Mestranol • Estradiol 17 beta-cypionate# • Polyestradiol phosphate) • Estrone (Estrone sulfate) • Estriol • Promestriene • Equilenin • EquilinAfimoxifene • Arzoxifene • Bazedoxifene • Cyclofenil • Lasofoxifene • Ormeloxifene • Raloxifene • Tamoxifen • Toremifenepure antagonist: Fulvestrant#WHO-EM. ‡Withdrawn from market. Clinical trials: †Phase III. §Never to phase III 
This drug article relating to the genito-urinary system is a stub. You can help Wikipedia by expanding it.
