- Drospirenone
-
Drospirenone 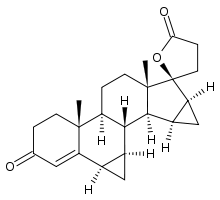
Systematic (IUPAC) name (6R,7R,8R,9S,10R,13S,14S,15S,16S,17S)-
1,3',4',6,6a,7,8,9,10,11,12,13,14,15,15a,16-
hexadecahydro-10,13-dimethylspiro-
[17H-dicyclopropa-6,7:15,16]cyclopenta
[a]phenanthrene-17,2'(5H)-furan]-3,5'(2H)-dione)Clinical data Pregnancy cat. X(US) Legal status ? Routes Oral Pharmacokinetic data Bioavailability 76% Protein binding 97% Metabolism Hepatic, minor (CYP3A4-mediated) Half-life 30 hours Excretion Renal and fecal Identifiers CAS number 67392-87-4 
ATC code G03AA12 PubChem CID 68873 DrugBank DB01395 ChemSpider 62105 
UNII N295J34A25 
KEGG D03917 
ChEBI CHEBI:50838 
ChEMBL CHEMBL1509 
Chemical data Formula C24H30O3 Mol. mass 366.493 g/mol SMILES eMolecules & PubChem  (what is this?) (verify)
(what is this?) (verify)Drospirenone is a synthetic progestin that is typically used in birth control. There have been concerns raised regarding its safety and is thus not recommended as a first line agent.[1]
Contents
Medical uses
Drospirenone is part of certain birth control formulations. The compound differs from other synthetic progestins in that its pharmacological profile in preclinical studies shows it to be closer to the natural progesterone. As such it has anti-mineralocorticoid properties, counteracts the estrogen-stimulated activity of the renin-angiotensin-aldosterone system, and is not androgenic. With its activities similar to spironolactone it may lead to less water retention and breast tenderness while improving skin appearance (less acne).
Drospirenone is taken orally with about 76% bioavailability. It is bound not by sex hormone-binding globulin or corticosteroid binding globulin, but by other serum proteins. Metabolites have not been shown to be biologically active, show up in urine and feces, and are essentially completely excreted within 10 days.
The compound is part of certain newer oral contraceptive formulations:
- Yasmin contains 3 mg drospirenone and 30 mcg ethinylestradiol per tablet. It is indicated for the prevention of pregnancy in women who elect an oral contraceptive.
- Yasminelle contains 3 mg drospirenone and 20 mcg ethinylestradiol per tablet and is used for contraception.
- Yaz contains 3 mg drospirenone and 20 mcg ethinylestradiol per tablet and is given for 24/4 days with the same indications.
It has also been formulated in medication to manage menopausal symptoms using 0.5 mg drsp and 1 mg estradiol per day by oral application. This medication was introduced in the United States in 2007 as Angeliq.
Drospirenone at 3 mg, which can potentially cause hyperkalemia in high-risk patients, is comparable to a 25 mg dose of spironolactone.
The medication is contraindicated in patients with hepatic dysfunction, renal insufficiency, adrenal insufficiency, or in whom the use of oral contraceptives is contraindicated, such as smokers and patients with a history of DVT, stroke, or other blood clots. Because of the anti-mineralocorticoid effects care needs to be exercised when other drugs that may increase potassium levels are taken. Such medications include ACE inhibitors, angiotensin-II receptor agonists, potassium-sparing diuretics, potassium supplementation, heparin, aldosterone antagonists, and NSAIDs.
Adverse effects
Drospirenone appears to increase the risk of venous thromboembolism more than levonorgestrel.[2]
On September 26, 2011 the U.S. Food and Drug Administration (FDA) announced that it has not yet reached a conclusion, but remains concerned, about the potential increased risk of blood clots with the use of drospirenone-containing birth control pills.Preliminary results of the FDA-funded study suggest an approximately 1.5-fold increase in the risk of blood clots for women who use drospirenone-containing birth control pills compared to users of other hormonal contraceptives.[3]Chemistry
It is an analog to spironolactone, with a molecular weight of 366.5 and the molecular formula C24H30O3.
Litigation
In 2008, a series of television commercials prompted the FDA to cite Bayer for overstating the approved uses of Yaz while failing to adequately address the risks of the drug. Bayer was required to dispel the inaccurate information contained in those ads by creating new ads that clarified the drug's approved uses.[4]
On October 8, 2009, Bayer disclosed that 129 lawsuits had been brought against them over the side effects and marketing of Yaz and Yasmin. The allegations include (but are not limited to):- Glossing over risks associated with the products and overstating their approved uses in an effort to mislead users of Yaz and Yasmin into believing that the drugs were safe.[4][5]
- Failure to properly research the medication.
- Failing to recall the drug after post-marketing reports demonstrated that the risk of potentially life-threatening side effects of Yasmin and Yaz outweighed potential benefits that could be achieved via other available oral contraceptives.
In September 2009, the FDA cited Bayer for sending out potentially low-quality batches of drosperinone. Bayer justified the shipments by explaining that they monitor the "average" quality of all shipments, not the quality of each individual batch.
In October 2011 the CBC TV program Market Place ran a segment discussing issues involved with the usage of Yaz/Yasmin [6]
See also
- Progestins
- Combined oral contraceptive pill
- Oral contraceptive formulations
- Hormone replacement therapy
References
- ^ http://www.bmj.com/content/342/bmj.d2519.extract
- ^ Dunn, N (2011 Apr 21). "The risk of deep venous thrombosis with oral contraceptives containing drospirenone.". BMJ (Clinical research ed.) 342: d2519. PMID 21511807.
- ^ http://www.fda.gov/Safety/MedWatch/SafetyInformation/SafetyAlertsforHumanMedicalProducts/ucm257337.htm
- ^ a b http://www.courthousenews.com/2009/10/15/Yasmin_YAZ_Claims_Against_Bayer_Mount_Up.htm
- ^ "Nurse Charges Dangerous Yaz Birth Control Drug "Altered My Life"". Reuters. October 21, 2009. http://www.reuters.com/article/pressRelease/idUS204695+21-Oct-2009+BW20091021.
- ^ http://www.cbc.ca/news/health/story/2011/01/20/yasmin-marketplace.html
- Krattenmacher R (2000). "Drospirenone: pharmacology and pharmacokinetics of a unique progestogen". Contraception 62 (1): 29–38. doi:10.1016/S0010-7824(00)00133-5. PMID 11024226.
- Yasmin product information
- Yaz product information
- Angeliq product information
Estrogens and progestogens (G03C-D, L02) Progestogens/
progestins
(progesterone)AgonistAndrostene (Drospirenone) • 19-norprogesterone (Nomegestrol • Promegestone • Trimegestone) • 19-nortestosterone (Dienogest)Other/
ungroupedPregnenedione (Gestonorone) • Pregnene (Ethisterone) • Pregnadiene (Medrogestone • Melengestrol) • Norpregnane (Norgestrienone) • Lynestrenol • Norethynodrel • Tibolone • Dydrogesterone • Quingestanolantagonist: MifepristoneEstrogens AgonistDiosgenin • Estradiol (Ethinylestradiol#/Mestranol • Estradiol 17 beta-cypionate# • Polyestradiol phosphate) • Estrone (Estrone sulfate) • Estriol • Promestriene • Equilenin • EquilinAfimoxifene • Arzoxifene • Bazedoxifene • Cyclofenil • Lasofoxifene • Ormeloxifene • Raloxifene • Tamoxifen • ToremifeneCategories:- Progestagens
- Enantiopure drugs
- Spiro compounds
- Cyclopropanes
- Lactones
Wikimedia Foundation. 2010.
