- Spironolactone
-
Spironolactone 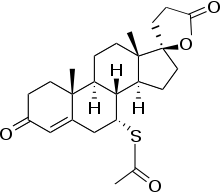
Systematic (IUPAC) name 7α-Acetylthio-3-oxo-17α-pregn-4-ene-21,17-carbolactone
OR
(1' S,2R,2' R,9' R,10' R,11' S,15' S)-9'-(acetylsulfanyl)-2',15'-dimethylspiro[oxolane-2,14'-tetracyclo[8.7.0.02,7.011,15]heptadecan]-6'-ene-5,5'-dioneClinical data Trade names Aldactone AHFS/Drugs.com monograph MedlinePlus a682627 Pregnancy cat. B3(AU) C(US) Legal status POM (UK) ℞-only (US) Routes Oral Pharmacokinetic data Metabolism Hepatic Half-life 1.3-2h Excretion Urine, bile Identifiers CAS number 52-01-7 
ATC code C03DA01 PubChem CID 5833 DrugBank APRD01234 ChemSpider 5628 
UNII 27O7W4T232 
KEGG D00443 
ChEBI CHEBI:9241 
ChEMBL CHEMBL1393 
Synonyms Aldactone
Spirotone
SpirolactoneChemical data Formula C24H32O4S Mol. mass 416.574 g/mol SMILES eMolecules & PubChem  (what is this?) (verify)
(what is this?) (verify)Spironolactone (marketed under the trade names Aldactone, Novo-Spiroton, Aldactazide, Spiractin, Spirotone, Verospiron or Berlactone), commonly referred to as simply spiro, is a diuretic and is used as an antiandrogen.
It is a synthetic 17-lactone drug that is a renal competitive aldosterone antagonist in a class of pharmaceuticals called potassium-sparing diuretics, used primarily to treat heart failure, ascites in patients with liver disease, low-renin hypertension, hypokalemia, secondary hyperaldosteronism (such as occurs with hepatic cirrhosis), and Conn's syndrome (primary hyperaldosteronism). On its own, spironolactone is only a weak diuretic because its effects target the distal nephron (collecting tubule), where urine volume can only be slightly modified; but it can be combined with other diuretics to increase efficacy. About one person in one hundred with hypertension has elevated levels of aldosterone; in these persons, the antihypertensive effect of spironolactone may exceed that of complex combined regimens of other antihypertensives.
Due to its antiandrogen effect, it can also be used to treat hirsutism. It is also used for treating hair loss and acne in women, and can be used as a topical medication for treatment of male baldness. It is commonly used to treat symptoms of polycystic ovary syndrome (PCOS) such as excess facial hair and acne. It can also be used as part of sex reassignment therapy by trans women.
Spironolactone can cause gynecomastia in males and, unless regularly monitored, should not be given with potassium supplementation for fear of development of hyperkalemia.
Contents
Mechanism of action
Spironolactone inhibits the effect of aldosterone by competing for intracellular aldosterone receptors in the cortical collecting duct. This decreases the reabsorption of sodium and water, while decreasing the secretion of potassium. Spironolactone has a fairly slow onset of action, taking several days to develop, and, so, the effect diminishes slowly. This is because steroid pathways alter gene transcription, and it will take several days for the gene products to change (in this case the ENaC and ROMK channels will be decreased).
Spironolactone has anti-androgen activity by directly binding to and blocking androgens from interacting with the androgen receptor, by blocking androgen production, and by increasing estrogen levels. Production of androgens is decreased by inhibiting 17α-hydroxylase and 17,20-desmolase, which are enzymes in the testosterone biosynthesis pathway. Estrogen levels are increased by enhancing the peripheral conversion of testosterone to estradiol and by displacing estradiol from sex hormone-binding globulin (SHBG).[1][2]
Pharmacokinetics
Spironolactone is a synthetic steroid that acts as a competitive antagonist to aldosterone. Its onset and duration of action are determined by the kinetics of the aldosterone response in the target tissue. Substantial inactivation of spironolactone occurs in the liver and hepatitis or cirrhosis can lead to secondary aldosteronism, which is one indication for treatment. Overall, spironolactone has a rather slow onset of action, requiring several days before full therapeutic effect is achieved. It may take up to 6 weeks to see full anti-hypertensive effects.
Mortality and morbidity benefit in heart failure
Spironolactone was shown to have a significant mortality and morbidity benefit in the Randomized Aldactone Evaluation Study (RALES), which studied people with severe congestive heart failure (New York Heart Association functional class III or IV).[3] Patients in the study arm of the trial (those receiving spironolactone) had a relative risk of death (when compared to the placebo group) equal to 0.70 or a 30% relative risk reduction. Patients in the study arm also had fewer symptoms of CHF and were hospitalized less frequently.
The mechanism of this effect is also mediated by inhibiting aldosterone, which in conjunction with heart failure leads to myocardial remodeling including fibrosis, sodium retention, and vascular dysfunction.
Adverse effects and interactions
Spironolactone is associated with an increased risk of bleeding from the stomach and duodenum, but a causal relationship between the two has not been established.[4] Because it also affects androgen receptors and other steroid receptors, it can cause gynecomastia, menstrual irregularities and testicular atrophy. Other side effects include ataxia, erectile dysfunction, drowsiness, and rashes. A carcinogenic effect has been demonstrated in rats, see below. Spironolactone has been shown to be immunosuppressive in the treatment of sarcoidosis.[5]
Spironolactone often increases serum potassium levels and can cause hyperkalemia, a very serious condition. Therefore, it is recommended that people using this drug avoid potassium supplements and salt substitutes containing potassium.[6] Doctors usually recommend periodic screening of serum potassium levels and some patients may be advised to limit dietary consumption of potassium.
Research has also shown spironolactone can interfere with the effectiveness of antidepressant treatment. The drug is actually (among its other receptor interactions) a mineralocorticoid (MR) antagonist, and has been found to reduce the effectiveness of antidepressant drugs in the treatment of major depression, it is presumed, by interfering with normalization of the hypothalamic-pituitary-adrenal axis in patients receiving antidepressant therapy.[7]
Carcinogenicity
Studies of spironolactone and the related compound potassium canrenoate (which, like spironolactone, metabolizes to canrenone) in rats for one- to two-year periods show an increase in carcinogenesis in the thyroid gland, testes, liver, breasts, and myelocytic leukocytes. Mammalian cells, depending on the presence of metabolic activation, show mixed results for mutagenicity in vitro.[8] Doses relative to body weight were 10 to 150 mg per kg, which is ten to 500 times higher than normal doses for treating humans. In light of this research, Sandoz has recommended that unnecessary use of spironolactone be avoided.
Other potential benefits
It has been suggested that spironolactone can reduce the risk of Alzheimer's disease. In one study,[9] researchers observed a reduction in the risk of Alzheimer's specifically associated with potassium-sparing diuretics. Unpublished findings from other studies, including the Gothenberg Study have suggested that higher potassium levels may be associated with a lower risk of dementia.
Spironolactone may have antifibrotic properties[citation needed] and an NIH-sponsored randomized control trial of treatment of patients with diastolic heart failure with this drug, known as the TOPCAT Study has been planned (heart failure patients with diastolic dysfunction have evidence of active collagen metabolism and increasing fibrosis[citation needed]).
Synthesis
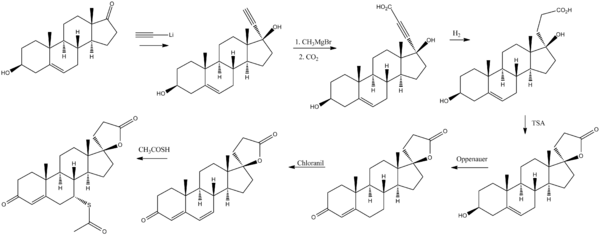 [10] (See also part 1 and part 3)
[10] (See also part 1 and part 3)Spironolactone bodies
Long-term administration of spironolactone gives the histologic characteristic of spironolactone bodies in the adrenal cortex. Spironolactone bodies are eosinophilic, round, concentrically laminated cytoplasmic inclusions surrounded by clear halos in preparations stained with hematoxylin and eosin.[11]
See also
References
- ^ Haynes BA, Mookadam F (August 2009). "Male gynecomastia". Mayo Clinic Proceedings. Mayo Clinic 84 (8): 672. doi:10.4065/84.8.672. PMC 2719518. PMID 19648382. http://www.mayoclinicproceedings.com/cgi/pmidlookup?view=long&pmid=19648382.
- ^ Donald W. Seldin; Gerhard H. Giebisch (4 September 1997). Diuretic agents: clinical physiology and pharmacology. Academic Press. pp. 630. ISBN 978-0-12-635690-8. http://books.google.com/books?id=VHcsrw6unuAC&pg=PA630. Retrieved 17 November 2011.
- ^ Pitt B, Zannad F, Remme W, Cody R, Castaigne A, Perez A, Palensky J, Wittes J (1999). "The effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized Aldactone Evaluation Study Investigators". N Engl J Med 341 (10): 709–17. doi:10.1056/NEJM199909023411001. PMID 10471456. http://content.nejm.org/cgi/content/full/341/10/709.
- ^ Verhamme KMC, Mosis G, Dieleman JP, et al. (2006). "Spironolactone and risk of upper gastrointestinal events: population based case-control study". Brit Med J 333 (7563): 330–3. doi:10.1136/bmj.38883.479549.2F. PMC 1539051. PMID 16840442. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=1539051.
- ^ Wandelt-Freerksen E. (1977). "Aldactone in the treatment of sarcoidosis of the lungs". JZ Erkr Atmungsorgane. 149 (1): 156–9. PMID 607621.
- ^ "Advisory Statement" (pdf). Klinge Chemicals / LoSalt. Archived from the original on 2006-11-15. http://web.archive.org/web/20061115185827/http://www.losalt.com/docs/lo_salt_web_advice.pdf. Retrieved 2007-03-15.
- ^ Holsboer, F. The Rationale for Corticotropin-Releasing Hormone Receptor (CRH-R) Antagonists to Treat Depression and Anxiety. J. Psychiatr. Res. 33, 181–214 (1999)
- ^ "Spironolactone RX Monograph". Sandoz Inc.. http://dailymed.nlm.nih.gov/dailymed/fdaDrugXsl.cfm?id=2479&type=display. Retrieved 2007-05-02.
- ^ [archneur.ama-assn.org/cgi/content/full/63/5/686 Antihypertensive Medication Use and Incident Alzheimer Disease]. Arch Neurol. 2006;63:686-692. Published online March 13, 2006 (doi:10.1001/archneur.63.5.noc60013).
- ^ Cella, John A.; Tweit, Robert C. (1959). Journal of Organic Chemistry 24 (8): 1109. doi:10.1021/jo01090a019.
- ^ Aiba M, Suzuki H, Kageyama K, et al. (June 1981). "Spironolactone bodies in aldosteronomas and in the attached adrenals. Enzyme histochemical study of 19 cases of primary aldosteronism and a case of aldosteronism due to bilateral diffuse hyperplasia of the zona glomerulosa". Am. J. Pathol. 103 (3): 404–10. PMC 1903848. PMID 7195152. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=1903848.
External links
Antihypertensives: diuretics (C03) Sulfonamides
(except EA)CA inhibitors (at PT)Thiazide-likes (primarily DCT)Quinethazone • Clopamide • Chlortalidone • Mefruside • Clofenamide • Metolazone • Meticrane • Xipamide • Indapamide • Clorexolone • FenquizonePotassium-sparing (at CD) ESC blockersOsmotic diuretics (PT, DL) VAs (DCT and CD) vaptans: Conivaptan • Mozavaptan • Satavaptan • Tolvaptan
tetracyclines: DemeclocyclineOther Androgens (G03B) Agonist Testosterone# • Androstanolone • Fluoxymesterone • Mesterolone • Methyltestosterone • see also Anabolic steroidsSARM AC-262,356§ • Andarine (S-4)§ • BMS-564,929§ • LGD-2226§ • LGD-3303§ • Ostarine (MK-2866)§ • S-23§ • S-40503§Antiandrogen Bicalutamide • Cyproterone • Dienogest • Flutamide • Galeterone§ • MDV3100† • Nilutamide • SpironolactoneEnzyme inhibitors Alfatradiol • Bexlosteride • Dutasteride • Epristeride • Finasteride • Izonsteride • Lapisteride • TurosterideCYP17A1 inhibitorsAbiraterone • Galeterone§ • KetoconazoleCategories:- Aldosterone antagonists
- Antiandrogens
- World Health Organization essential medicines
- Furones
- Spiro compounds
Wikimedia Foundation. 2010.

