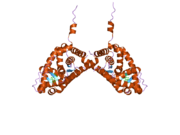- Mineralocorticoid receptor
-
The mineralocorticoid receptor (or MR, MLR, MCR), also known as the aldosterone receptor or nuclear receptor subfamily 3, group C, member 2, (NR3C2) is a protein that in humans is encoded by the NR3C2 gene that is located on chromosome 4q31.1-31.2.[1]
MR is a receptor with high affinity for mineralocorticoids. It belongs to the nuclear receptor family where the ligand diffuses into cells, interacts with the receptor and results in a signal transduction affecting specific gene expression in the nucleus.
Contents
Function
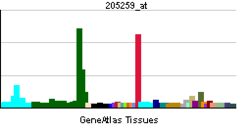
MR is expressed in many tissues, such as the kidney, colon, heart, central nervous system (hippocampus), brown adipose tissue and sweat glands. In epithelial tissues, its activation leads to the expression of proteins regulating ionic and water transports (mainly the epithelial sodium channel or ENaC, Na+/K+ pump, serum and glucocorticoid induced kinase or SGK1) resulting in the reabsoprtion of sodium, and as a consequence an increase in extracellular volume, increase in blood pressure, and an excretion of potassium to maintain a normal salt concentration in the body.
The receptor is activated by mineralocorticoids such as aldosterone and deoxycorticosterone as well as glucocorticoids, like cortisol. In intact animals, the mineralocorticoid receptor is "protected" from glucocorticoids by co-localization of an enzyme, 11ß-hydroxysteroid dehydrogenase type 2 (11ß-HSD2), that converts cortisol to inactive cortisone. It also responds to some progestins. Spironolactone and eplerenone are mineralocorticoid receptor antagonists.
Activation of the mineralocorticoid receptor, upon the binding of its ligand aldosterone, results in its translocation to the cell nucleus, homodimerization and binding to hormone response elements present in the promoter of some genes. This results in the complex recruitment of the transcriptional machinery and the transcription into mRNA of the DNA sequence of the activated genes.[2]
Interactions
Mineralocorticoid receptor has been shown to interact with:
References
- ^ Fan YS, Eddy RL, Byers MG, Haley LL, Henry WM, Nowak NJ, Shows TB (1989). "The human mineralocorticoid receptor gene (MLR) is located on chromosome 4 at q31.2". Cytogenet. Cell Genet. 52 (1-2): 83–4. doi:10.1159/000132846. PMID 2558856.
- ^ Fuller PJ, Young MJ (2005). "Mechanisms of mineralocorticoid action". Hypertension 46 (6): 1227–35. doi:10.1161/01.HYP.0000193502.77417.17. PMID 16286565.
- ^ a b Savory, J G; Préfontaine G G, Lamprecht C, Liao M, Walther R F, Lefebvre Y A, Haché R J (Feb. 2001). "Glucocorticoid receptor homodimers and glucocorticoid-mineralocorticoid receptor heterodimers form in the cytoplasm through alternative dimerization interfaces". Mol. Cell. Biol. (UNITED STATES) 21 (3): 781–93. doi:10.1128/MCB.21.3.781-793.2001. ISSN 0270-7306. PMC 86670. PMID 11154266. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=86670.
- ^ Zennaro MC, Souque A, Viengchareun S, Poisson E, Lombès M (September 2001). "A new human MR splice variant is a ligand-independent transactivator modulating corticosteroid action". Mol. Endocrinol. 15 (9): 1586–98. doi:10.1210/me.15.9.1586. PMID 11518808.
- ^ Thénot S, Henriquet C, Rochefort H, Cavaillès V (May 1997). "Differential interaction of nuclear receptors with the putative human transcriptional coactivator hTIF1". J. Biol. Chem. 272 (18): 12062–8. doi:10.1074/jbc.272.18.12062. PMID 9115274.
Further reading
- Hellal-Levy C, Fagart J, Souque A, Rafestin-Oblin ME (2000). "Mechanistic aspects of mineralocorticoid receptor activation.". Kidney Int. 57 (4): 1250–5. doi:10.1046/j.1523-1755.2000.00958.x. PMID 10760050.
- Sheppard KE (2002). "Nuclear receptors. II. Intestinal corticosteroid receptors.". Am. J. Physiol. Gastrointest. Liver Physiol. 282 (5): G742–6. doi:10.1152/ajpgi.00531.2001. PMID 11960770.
- Kjellander CG (1975). "[The psychotherapeutic society--utopia or nightmare?]". Lakartidningen 72 (12): 1160–1. PMID 1134129.
- Alnemri ES, Maksymowych AB, Robertson NM, Litwack G (1991). "Overexpression and characterization of the human mineralocorticoid receptor.". J. Biol. Chem. 266 (27): 18072–81. PMID 1655735.
- Morrison N, Harrap SB, Arriza JL, et al. (1990). "Regional chromosomal assignment of the human mineralocorticoid receptor gene to 4q31.1.". Hum. Genet. 85 (1): 130–2. doi:10.1007/BF00276340. PMID 2162806.
- Fan YS, Eddy RL, Byers MG, et al. (1990). "The human mineralocorticoid receptor gene (MLR) is located on chromosome 4 at q31.2.". Cytogenet. Cell Genet. 52 (1-2): 83–4. doi:10.1159/000132846. PMID 2558856.
- Arriza JL, Weinberger C, Cerelli G, et al. (1987). "Cloning of human mineralocorticoid receptor complementary DNA: structural and functional kinship with the glucocorticoid receptor.". Science 237 (4812): 268–75. doi:10.1126/science.3037703. PMID 3037703.
- Bloem LJ, Guo C, Pratt JH (1996). "Identification of a splice variant of the rat and human mineralocorticoid receptor genes.". J. Steroid Biochem. Mol. Biol. 55 (2): 159–62. doi:10.1016/0960-0760(95)00162-S. PMID 7495694.
- Jalaguier S, Mornet D, Mesnier D, et al. (1996). "Human mineralocorticoid receptor interacts with actin under mineralocorticoid ligand modulation.". FEBS Lett. 384 (2): 112–6. doi:10.1016/0014-5793(96)00295-5. PMID 8612804.
- Thénot S, Henriquet C, Rochefort H, Cavaillès V (1997). "Differential interaction of nuclear receptors with the putative human transcriptional coactivator hTIF1.". J. Biol. Chem. 272 (18): 12062–8. doi:10.1074/jbc.272.18.12062. PMID 9115274.
- Zennaro MC, Farman N, Bonvalet JP, Lombès M (1997). "Tissue-specific expression of alpha and beta messenger ribonucleic acid isoforms of the human mineralocorticoid receptor in normal and pathological states.". J. Clin. Endocrinol. Metab. 82 (5): 1345–52. doi:10.1210/jc.82.5.1345. PMID 9141514.
- Bruner KL, Derfoul A, Robertson NM, et al. (1998). "The unliganded mineralocorticoid receptor is associated with heat shock proteins 70 and 90 and the immunophilin FKBP-52.". Receptors & signal transduction 7 (2): 85–98. PMID 9392437.
- Geller DS, Rodriguez-Soriano J, Vallo Boado A, et al. (1998). "Mutations in the mineralocorticoid receptor gene cause autosomal dominant pseudohypoaldosteronism type I.". Nat. Genet. 19 (3): 279–81. doi:10.1038/966. PMID 9662404.
- Lupo B, Mesnier D, Auzou G (1998). "Cysteines 849 and 942 of human mineralocorticoid receptor are crucial for steroid binding.". Biochemistry 37 (35): 12153–9. doi:10.1021/bi980593e. PMID 9724527.
- Halushka MK, Fan JB, Bentley K, et al. (1999). "Patterns of single-nucleotide polymorphisms in candidate genes for blood-pressure homeostasis.". Nat. Genet. 22 (3): 239–47. doi:10.1038/10297. PMID 10391210.
- Freeman BC, Felts SJ, Toft DO, Yamamoto KR (2000). "The p23 molecular chaperones act at a late step in intracellular receptor action to differentially affect ligand efficacies.". Genes Dev. 14 (4): 422–34. PMC 316379. PMID 10691735. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=316379.
- Geller DS, Farhi A, Pinkerton N, et al. (2000). "Activating mineralocorticoid receptor mutation in hypertension exacerbated by pregnancy.". Science 289 (5476): 119–23. doi:10.1126/science.289.5476.119. PMID 10884226.
- Hellal-Levy C, Fagart J, Souque A, et al. (2001). "Crucial role of the H11-H12 loop in stabilizing the active conformation of the human mineralocorticoid receptor.". Mol. Endocrinol. 14 (8): 1210–21. doi:10.1210/me.14.8.1210. PMID 10935545.
- Watzka M, Beyenburg S, Blümcke I, et al. (2000). "Expression of mineralocorticoid and glucocorticoid receptor mRNA in the human hippocampus.". Neurosci. Lett. 290 (2): 121–4. doi:10.1016/S0304-3940(00)01325-2. PMID 10936692.
External links
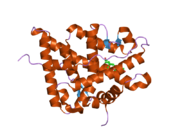
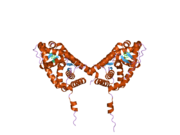
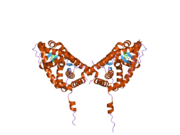
PDB gallery 1gdc: REFINED SOLUTION STRUCTURE OF THE GLUCOCORTICOID RECEPTOR DNA-BINDING DOMAIN1rgd: STRUCTURE REFINEMENT OF THE GLUCOCORTICOID RECEPTOR-DNA BINDING DOMAIN FROM NMR DATA BY RELAXATION MATRIX CALCULATIONS1y9r: Crystal structure of the human mineralocorticoid receptor ligand-binding domain bound to deoxycorticosterone and harboring the S810L mutation responsible for a severe form of hypertension1ya3: Crystal structure of the human mineralocorticoid receptor ligand-binding domain bound to progesterone and harboring the S810L mutation responsible for a severe form of hypertension2a3i: Structural and Biochemical Mechanisms for the Specificity of Hormone Binding and Coactivator Assembly by Mineralocorticoid Receptor2aa2: Mineralocorticoid Receptor with Bound Aldosterone2aa5: Mineralocorticoid Receptor with Bound Progesterone2aa6: Mineralocorticoid Receptor S810L Mutant with Bound Progesterone2aa7: Mineralocorticoid Receptor with Bound Deoxycorticosterone2aax: Mineralocorticoid Receptor Double Mutant with Bound Cortisone2ab2: Mineralocorticoid Receptor Double Mutant with Bound Spironolactone2abi: Crystal structure of the human mineralocorticoid receptor ligand-binding domain bound to deoxycorticosterone2gda: REFINED SOLUTION STRUCTURE OF THE GLUCOCORTICOID RECEPTOR DNA-BINDING DOMAINTranscription factors and intracellular receptors (1) Basic domains (1.1) Basic leucine zipper (bZIP)Activating transcription factor (AATF, 1, 2, 3, 4, 5, 6, 7) · AP-1 (c-Fos, FOSB, FOSL1, FOSL2, JDP2, c-Jun, JUNB, JUND) · BACH (1, 2) · BATF · BLZF1 · C/EBP (α, β, γ, δ, ε, ζ) · CREB (1, 3, L1) · CREM · DBP · DDIT3 · GABPA · HLF · MAF (B, F, G, K) · NFE (2, L1, L2, L3) · NFIL3 · NRL · NRF (1, 2, 3) · XBP1(1.2) Basic helix-loop-helix (bHLH)ATOH1 · AhR · AHRR · ARNT · ASCL1 · BHLHB2 · BMAL (ARNTL, ARNTL2) · CLOCK · EPAS1 · FIGLA · HAND (1, 2) · HES (5, 6) · HEY (1, 2, L) · HES1 · HIF (1A, 3A) · ID (1, 2, 3, 4) · LYL1 · MESP2 · MXD4 · MYCL1 · MYCN · Myogenic regulatory factors (MyoD, Myogenin, MYF5, MYF6) · Neurogenins (1, 2, 3) · NeuroD (1, 2) · NPAS (1, 2, 3) · OLIG (1, 2) · Pho4 · Scleraxis · SIM (1, 2) · TAL (1, 2) · Twist · USF1(1.3) bHLH-ZIP(1.4) NF-1(1.5) RF-X(1.6) Basic helix-span-helix (bHSH)(2) Zinc finger DNA-binding domains (2.1) Nuclear receptor (Cys4)subfamily 1 (Thyroid hormone (α, β), CAR, FXR, LXR (α, β), PPAR (α, β/δ, γ), PXR, RAR (α, β, γ), ROR (α, β, γ), Rev-ErbA (α, β), VDR)
subfamily 2 (COUP-TF (I, II), Ear-2, HNF4 (α, γ), PNR, RXR (α, β, γ), Testicular receptor (2, 4), TLX)
subfamily 3 (Steroid hormone (Androgen, Estrogen (α, β), Glucocorticoid, Mineralocorticoid, Progesterone), Estrogen related (α, β, γ))
subfamily 4 NUR (NGFIB, NOR1, NURR1) · subfamily 5 (LRH-1, SF1) · subfamily 6 (GCNF) · subfamily 0 (DAX1, SHP)(2.2) Other Cys4(2.3) Cys2His2General transcription factors (TFIIA, TFIIB, TFIID, TFIIE (1, 2), TFIIF (1, 2), TFIIH (1, 2, 4, 2I, 3A, 3C1, 3C2))
ATBF1 · BCL (6, 11A, 11B) · CTCF · E4F1 · EGR (1, 2, 3, 4) · ERV3 · GFI1 · GLI-Krüppel family (1, 2, 3, REST, S2, YY1) · HIC (1, 2) · HIVEP (1, 2, 3) · IKZF (1, 2, 3) · ILF (2, 3) · KLF (2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 17) · MTF1 · MYT1 · OSR1 · PRDM9 · SALL (1, 2, 3, 4) · SP (1, 2, 4, 7, 8) · TSHZ3 · WT1 · Zbtb7 (7A, 7B) · ZBTB (16, 17, 20, 32, 33, 40) · zinc finger (3, 7, 9, 10, 19, 22, 24, 33B, 34, 35, 41, 43, 44, 51, 74, 143, 146, 148, 165, 202, 217, 219, 238, 239, 259, 267, 268, 281, 295, 300, 318, 330, 346, 350, 365, 366, 384, 423, 451, 452, 471, 593, 638, 644, 649, 655)(2.4) Cys6(2.5) Alternating composition(3) Helix-turn-helix domains (3.1) HomeodomainARX · CDX (1, 2) · CRX · CUTL1 · DBX (1, 2) · DLX (3, 4, 5) · EMX2 · EN (1, 2) · FHL (1, 2, 3) · HESX1 · HHEX · HLX · Homeobox (A1, A2, A3, A4, A5, A7, A9, A10, A11, A13, B1, B2, B3, B4, B5, B6, B7, B8, B9, B13, C4, C5, C6, C8, C9, C10, C11, C12, C13, D1, D3, D4, D8, D9, D10, D11, D12, D13) · HOPX · IRX (1, 2, 3, 4, 5, 6, MKX) · LMX (1A, 1B) · MEIS (1, 2) · MEOX2 · MNX1 · MSX (1, 2) · NANOG · NKX (2-1, 2-2, 2-3, 2-5, 3-1, 3-2, 6-1, 6-2) · NOBOX · PBX (1, 2, 3) · PHF (1, 3, 6, 8, 10, 16, 17, 20, 21A) · PHOX (2A, 2B) · PITX (1, 2, 3) · POU domain (PIT-1, BRN-3: A, B, C, Octamer transcription factor: 1, 2, 3/4, 6, 7, 11) · OTX (1, 2) · PDX1 · SATB2 · SHOX2 · VAX1 · ZEB (1, 2)(3.2) Paired box(3.3) Fork head / winged helix(3.4) Heat Shock Factors(3.5) Tryptophan clusters(3.6) TEA domain(4) β-Scaffold factors with minor groove contacts (4.1) Rel homology region(4.2) STAT(4.3) p53(4.4) MADS box(4.6) TATA binding proteins(4.7) High-mobility group(4.10) Cold-shock domainCSDA, YBX1(4.11) Runt(0) Other transcription factors (0.2) HMGI(Y)(0.3) Pocket domain(0.6) Miscellaneoussee also transcription factor/coregulator deficiencies
B bsyn: dna (repl, cycl, reco, repr) · tscr (fact, tcrg, nucl, rnat, rept, ptts) · tltn (risu, pttl, nexn) · dnab, rnab/runp · stru (domn, 1°, 2°, 3°, 4°)Corticosteroids – glucocorticoids and mineralocorticoids (H02)
(also A07EA, C05AA, D07, D10AA, R01AD, R03BA, S01BA, S02B, and S03B)Mineralocorticoids
(3-one, 4-ene,
no FG at 16)Glucocorticoids
(3-one, 4-ene,
11-FG,
17-hydroxy)PregnenePregnenedione
(+20-one)HYDROCORTISONE/CORTISOL# (Hydrocortisone aceponate, Hydrocortisone buteprate, Hydrocortisone butyrate) • Budesonide • Ciclesonide • Deflazacort • Medrysone • Tixocortol • HALOGENATED AT 6: Cloprednol • HALOGENATED, WITH FG AT 16: HalcinonidePregnadiene (+1-ene)Rimexolone • HALOGENATED, WITH FG AT 16: Flunisolide • Triamcinolone • Amcinonide • Fluocinolone acetonide (Fluocinonide)Pregnadienediol
(+21-hydroxy)Prednisone (Meprednisone) • HALOGENATED AT 9: Fluorometholone • HALOGENATED, WITH FG AT 16: Fluocortolone (Clocortolone, Diflucortolone, Fluocortin) • DesoximetasonePregnadienetriol
(+11-hydroxy)Prednisolone# (Methylprednisolone, Methylprednisolone aceponate, Prednicarbate, Prednylidene) • Desonide • HALOGENATED: Fluprednisolone (Difluprednate, Fluperolone) • HALOGENATED, WITH FG AT 16: Dexamethasone# • Betamethasone (Clobetasol, Clobetasone, Diflorasone, Halometasone, Ulobetasol) • Beclometasone • Paramethasone • Alclometasone • Fluclorolone • Flumetasone • FluprednidenePregnatriene
(+2-ene)HALOGENATED, WITH FG AT 16: Fluticasone (Fluticasone propionate, Fluticasone furoate)Other/ungroupedHALOGENATED: Loteprednol • HALOGENATED, WITH FG AT 16: Fludroxycortide • Formocortal • Mometasone furoateAldosterone antagonists Synthesis modifiers Categories:- Human proteins
- Intracellular receptors
- Transcription factors
Wikimedia Foundation. 2010.