- Rev-ErbA alpha
-
Nuclear receptor subfamily 1, group D, member 1 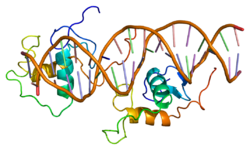
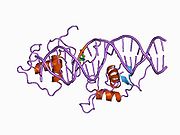
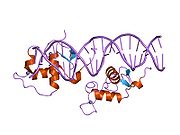
The DNA binding domain of NR1D1 bound to double stranded DNA.[1]Available structures PDB 1a6y, 1ga5, 1hlz, 3N00 Identifiers Symbols NR1D1; THRA1; EAR1; THRAL; ear-1; hRev External IDs OMIM: 602408 MGI: 2444210 HomoloGene: 23324 IUPHAR: NR1D1 GeneCards: NR1D1 Gene Gene Ontology Molecular function • transcription factor activity
• steroid hormone receptor activity
• transcription corepressor activity
• zinc ion binding
• sequence-specific DNA binding
• metal ion bindingCellular component • nucleus Biological process • transcription
• regulation of transcription, DNA-dependent
• circadian rhythmSources: Amigo / QuickGO RNA expression pattern 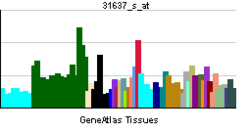
More reference expression data Orthologs Species Human Mouse Entrez 9572 217166 Ensembl ENSG00000126368 ENSMUSG00000020889 UniProt P20393 Q3UV55 RefSeq (mRNA) NM_021724 NM_145434 RefSeq (protein) NP_068370 NP_663409 Location (UCSC) Chr 17:
35.5 – 35.51 MbChr 11:
98.58 – 98.59 MbPubMed search [1] [2] This box: view · protein that in humans is encoded by the NR1D1 gene.[2] Rev-erbα is member of the Rev-ErbA family of nuclear receptors and is a transcriptional repressor.[3] In mammals, Rev-erbα is highly expressed in the liver, skeletal muscle, adipose tissue, and the brain, participating in the development and circadian regulation of these tissues.[4][5]
Contents
Gene and protein structure
Rev-erbα is transcribed from the opposite strand of the thyroid receptor α (c-erbAα) gene on chromosome 17, with a 269-nucleotide overlap between the two transcripts.[3] The other mammalian isoform of the receptor, Rev-erbβ is encoded by another gene on chromosome 3. In addition, there is one non-mammalian homolog, the ecdysone-regulated gene E75, which is present in Drosophila and C. elegans. The Rev-erbα gene itself has multiple transcripts. Two promoters govern the expression of the Rev-erbα gene in human and rat, generating two mRNA isoforms. The full-length isoform encodes a 614-amino acid protein, while a second isoform is generated from an internal promoter and produces a protein that is shorter by 106 amino acids. Both Rev-erbα mRNA isoforms contain E-boxes as well as Rev-erbα response elements, which means that they can be regulated in a circadian manner by the BMAL and Rev-erba proteins. In fact, both transcripts exhibit rhythmic expression in serum-synchronized fibroblasts.
The Rev-erbα protein is structurally unique from other nuclear receptors, in that it lacks helix 12 (H12) in its ligand-binding domain, which is usually responsible for forming the ligand binding pocket in other nuclear receptors. In place of the missing H12, Rev-erbα displays a hydrophobic interface that binds the corepressor N-CoR, making it a potent transcriptional repressor.[6] Interestingly, all members of the Rev-erb family bind heme, which may act as a ligand to regulate their transcriptional activity.[7]
Physiologic function
Rev-erbα regulates gene transcription by directly binding to target response elements (RevREs), comprises an A/T-rich flank followed by AGGTCA. Rev-erbα mediates repression by recruiting the corepressor N-CoR, which then activates the histone deacetylase (HDAC) 3. A number of target genes has been identified for Rev-erbα, including the lipoproteins ApoA1 and ApoCIII, hydratase dehydrogenase, the circadian factor BMAL, and the anti-fibrinolytic factor PAI-1.[8] Many of these genes are coordinately regulated by Rev-erbα and the RAR-related orphan receptor RORα, which share the same response elements but exert opposite effects on gene transcription. Crosstalk between Rev-erbα and RORα likely acts to fine-tune of their target physiologic networks, such as circadian rhythms, metabolic homeostasis, and inflammation.[9]
Rev-erbα mRNA is induced during adipogenesis and is highly expressed in adipose tissue.[10] One study reported that overexpression of Rev-erbα may enhance adipogenesis in cultured mouse adipocytes, but the mechanism of this effect remains to be elucidated.[11] Rev-erbα expression is also regulated at the post-translational level: it is phosphorylated on the amino terminus by glycogen synthase kinase (GSK 3β), which contributes to its protein stability. It has been shown that lithium, which inhibits GSK3β, can de-stabilize Rev-erbα protein and affect its function in the circadian clock.[12] This may partly explain lithium’s therapeutic effect on circadian diseases such as bipolar disorder.
References
- ^ PDB 1a6yZhao Q, Khorasanizadeh S, Miyoshi Y, Lazar MA, Rastinejad F (May 1998). "Structural elements of an orphan nuclear receptor-DNA complex". Mol. Cell 1 (6): 849–61. doi:10.1016/S1097-2765(00)80084-2. PMID 9660968.
- ^ Lazar MA, Jones KE, Chin WW (March 1990). "Isolation of a cDNA encoding human Rev-ErbA alpha: transcription from the noncoding DNA strand of a thyroid hormone receptor gene results in a related protein that does not bind thyroid hormone". DNA Cell Biol. 9 (2): 77–83. doi:10.1089/dna.1990.9.77. PMID 1971514.
- ^ a b Lazar MA, Hodin RA, Cardona G, Chin WW (1990). "Gene expression from the c-erbA α/Rev-ErbA α genomic locus. Potential regulation of alternative splicing by opposite strand transcription". J. Biol. Chem. 265 (22): 12859–63. PMID 2165488. http://www.jbc.org/cgi/content/abstract/265/22/12859.
- ^ Preitner N, Damiola F, Lopez-Molina L, Zakany J, Duboule D, Albrecht U, Schibler U (2002). "The orphan nuclear receptor REV-ERBα controls circadian transcription within the positive limb of the mammalian circadian oscillator". Cell 110 (2): 251–60. doi:10.1016/S0092-8674(02)00825-5. PMID 12150932.
- ^ Triqueneaux G, Thenot S, Kakizawa T, Antoch MP, Safi R, Takahashi JS, Delaunay F, Laudet V (2004). "The orphan receptor Rev-erbα gene is a target of the circadian clock pacemaker". J. Mol. Endocrinol. 33 (3): 585–608. doi:10.1677/jme.1.01554. PMID 15591021.
- ^ Woo EJ, Jeong DG, Lim MY, Jun Kim S, Kim KJ, Yoon SM, Park BC, Eon Ryu S (2007). "Structural Insight into the Constitutive Repression Function of the Nuclear Receptor Rev-erbβ". J. Mol. Biol. 373 (3): 735–44. doi:10.1016/j.jmb.2007.08.037. PMID 17870090.
- ^ Raghuram S, Stayrook KR, Huang P, Rogers PM, Nosie AK, McClure DB, Burris LL, Khorasanizadeh S, Burris TP, Rastinejad F (December 2007). "Identification of heme as the ligand for the orphan nuclear receptors REV-ERBα and REV-ERBβ". Nat. Struct. Mol. Biol. 14 (12): 1207–13. doi:10.1038/nsmb1344. PMC 2743565. PMID 18037887. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2743565.
- ^ Wang J, Yin L, Lazar MA (2006). "The orphan nuclear receptor Rev-erb α regulates circadian expression of plasminogen activator inhibitor type 1". J. Biol. Chem. 281 (45): 33842–8. doi:10.1074/jbc.M607873200. PMID 16968709.
- ^ Forman BM, Chen J, Blumberg B, Kliewer SA, Henshaw R, Ong ES, Evans RM (1994). "Cross-talk among ROR α 1 and the Rev-erb family of orphan nuclear receptors". Mol. Endocrinol. 8 (9): 1253–61. doi:10.1210/me.8.9.1253. PMID 7838158.
- ^ Fontaine C, Dubois G, Duguay Y, Helledie T, Vu-Dac N, Gervois P, Soncin F, Mandrup S, Fruchart JC, Fruchart-Najib J, Staels B (2003). "The orphan nuclear receptor Rev-Erbα is a peroxisome proliferator-activated receptor (PPAR) gamma target gene and promotes PPARgamma-induced adipocyte differentiation". J. Biol. Chem. 278 (39): 37672–80. doi:10.1074/jbc.M304664200. PMID 12821652.
- ^ Chawla A, Lazar MA (1993). "Induction of Rev-ErbA α, an orphan receptor encoded on the opposite strand of the α-thyroid hormone receptor gene, during adipocyte differentiation". J. Biol. Chem. 268 (22): 16265–9. PMID 8344913. http://www.jbc.org/cgi/content/abstract/268/22/16265.
- ^ Yin L, Wang J, Klein PS, Lazar MA (2006). "Nuclear receptor Rev-erbα is a critical lithium-sensitive component of the circadian clock". Science 311 (5763): 1002–5. doi:10.1126/science.1121613. PMID 16484495.
Further reading
- Laudet V, Begue A, Henry-Duthoit C et al. (1991). "Genomic organization of the human thyroid hormone receptor alpha (c-erbA-1) gene". Nucleic Acids Res. 19 (5): 1105–12. doi:10.1093/nar/19.5.1105. PMC 333788. PMID 1850510. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=333788.
- Miyajima N, Horiuchi R, Shibuya Y et al. (1989). "Two erbA homologs encoding proteins with different T3 binding capacities are transcribed from opposite DNA strands of the same genetic locus". Cell 57 (1): 31–9. doi:10.1016/0092-8674(89)90169-4. PMID 2539258.
- Adelmant G, Bègue A, Stéhelin D, Laudet V (1996). "A functional Rev-erb alpha responsive element located in the human Rev-erb alpha promoter mediates a repressing activity". Proc. Natl. Acad. Sci. U.S.A. 93 (8): 3553–8. doi:10.1073/pnas.93.8.3553. PMC 39648. PMID 8622974. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=39648.
- Downes M, Burke LJ, Bailey PJ, Muscat GE (1997). "Two receptor interaction domains in the corepressor, N-CoR/RIP13, are required for an efficient interaction with Rev-erbA alpha and RVR: physical association is dependent on the E region of the orphan receptors". Nucleic Acids Res. 24 (22): 4379–86. doi:10.1093/nar/24.22.4379. PMC 146280. PMID 8948627. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=146280.
- Burke LJ, Downes M, Laudet V, Muscat GE (1998). "Identification and characterization of a novel corepressor interaction region in RVR and Rev-erbA alpha". Mol. Endocrinol. 12 (2): 248–62. doi:10.1210/me.12.2.248. PMID 9482666.
- Zhao Q, Khorasanizadeh S, Miyoshi Y et al. (1998). "Structural elements of an orphan nuclear receptor-DNA complex". Mol. Cell 1 (6): 849–61. doi:10.1016/S1097-2765(00)80084-2. PMID 9660968.
- Sierk ML, Zhao Q, Rastinejad F (2001). "DNA deformability as a recognition feature in the reverb response element". Biochemistry 40 (43): 12833–43. doi:10.1021/bi011086r. PMID 11669620.
- Coste H, Rodríguez JC (2002). "Orphan nuclear hormone receptor Rev-erbalpha regulates the human apolipoprotein CIII promoter". J. Biol. Chem. 277 (30): 27120–9. doi:10.1074/jbc.M203421200. PMID 12021280.
- Delerive P, Chin WW, Suen CS (2002). "Identification of Reverb(alpha) as a novel ROR(alpha) target gene". J. Biol. Chem. 277 (38): 35013–8. doi:10.1074/jbc.M202979200. PMID 12114512.
- Raspè E, Mautino G, Duval C et al. (2003). "Transcriptional regulation of human Rev-erbalpha gene expression by the orphan nuclear receptor retinoic acid-related orphan receptor alpha". J. Biol. Chem. 277 (51): 49275–81. doi:10.1074/jbc.M206215200. PMID 12377782.
- Raspé E, Duez H, Mansén A et al. (2003). "Identification of Rev-erbalpha as a physiological repressor of apoC-III gene transcription". J. Lipid Res. 43 (12): 2172–9. doi:10.1194/jlr.M200386-JLR200. PMID 12454280.
- Strausberg RL, Feingold EA, Grouse LH et al. (2003). "Generation and initial analysis of more than 15,000 full-length human and mouse cDNA sequences". Proc. Natl. Acad. Sci. U.S.A. 99 (26): 16899–903. doi:10.1073/pnas.242603899. PMC 139241. PMID 12477932. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=139241.
- Chopin-Delannoy S, Thénot S, Delaunay F et al. (2004). "A specific and unusual nuclear localization signal in the DNA binding domain of the Rev-erb orphan receptors". J. Mol. Endocrinol. 30 (2): 197–211. doi:10.1677/jme.0.0300197. PMID 12683943.
- Fontaine C, Dubois G, Duguay Y et al. (2003). "The orphan nuclear receptor Rev-Erbalpha is a peroxisome proliferator-activated receptor (PPAR) gamma target gene and promotes PPARgamma-induced adipocyte differentiation". J. Biol. Chem. 278 (39): 37672–80. doi:10.1074/jbc.M304664200. PMID 12821652.
- Johnson JM, Castle J, Garrett-Engele P et al. (2004). "Genome-wide survey of human alternative pre-mRNA splicing with exon junction microarrays". Science 302 (5653): 2141–4. doi:10.1126/science.1090100. PMID 14684825.
- Migita H, Morser J, Kawai K (2004). "Rev-erbalpha upregulates NF-kappaB-responsive genes in vascular smooth muscle cells". FEBS Lett. 561 (1–3): 69–74. doi:10.1016/S0014-5793(04)00118-8. PMID 15013753.
- Cheng H, Khanna H, Oh EC et al. (2005). "Photoreceptor-specific nuclear receptor NR2E3 functions as a transcriptional activator in rod photoreceptors". Hum. Mol. Genet. 13 (15): 1563–75. doi:10.1093/hmg/ddh173. PMID 15190009.
- Beausoleil SA, Jedrychowski M, Schwartz D et al. (2004). "Large-scale characterization of HeLa cell nuclear phosphoproteins". Proc. Natl. Acad. Sci. U.S.A. 101 (33): 12130–5. doi:10.1073/pnas.0404720101. PMC 514446. PMID 15302935. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=514446.
- Gerhard DS, Wagner L, Feingold EA et al. (2004). "The Status, Quality, and Expansion of the NIH Full-Length cDNA Project: The Mammalian Gene Collection (MGC)". Genome Res. 14 (10B): 2121–7. doi:10.1101/gr.2596504. PMC 528928. PMID 15489334. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=528928.
External links
v · d · ePDB gallery 1a6y: REVERBA ORPHAN NUCLEAR RECEPTOR/DNA COMPLEX1ga5: CRYSTAL STRUCTURE OF THE ORPHAN NUCLEAR RECEPTOR REV-ERB(ALPHA) DNA-BINDING DOMAIN BOUND TO ITS COGNATE RESPONSE ELEMENT1hlz: CRYSTAL STRUCTURE OF THE ORPHAN NUCLEAR RECEPTOR REV-ERB(ALPHA) DNA-BINDING DOMAIN BOUND TO ITS COGNATE RESPONSE ELEMENTv · d · eTranscription factors and intracellular receptors (1) Basic domains Activating transcription factor (AATF, 1, 2, 3, 4, 5, 6, 7) · AP-1 (c-Fos, FOSB, FOSL1, FOSL2, JDP2, c-Jun, JUNB, JUND) · BACH (1, 2) · BATF · BLZF1 · C/EBP (α, β, γ, δ, ε, ζ) · CREB (1, 3, L1) · CREM · DBP · DDIT3 · GABPA · HLF · MAF (B, F, G, K) · NFE (2, L1, L2, L3) · NFIL3 · NRL · NRF (1, 2, 3) · XBP1ATOH1 · AhR · AHRR · ARNT · ASCL1 · BHLHB2 · BMAL (ARNTL, ARNTL2) · CLOCK · EPAS1 · FIGLA · HAND (1, 2) · HES (5, 6) · HEY (1, 2, L) · HES1 · HIF (1A, 3A) · ID (1, 2, 3, 4) · LYL1 · MESP2 · MXD4 · MYCL1 · MYCN · Myogenic regulatory factors (MyoD, Myogenin, MYF5, MYF6) · Neurogenins (1, 2, 3) · NeuroD (1, 2) · NPAS (1, 2, 3) · OLIG (1, 2) · Pho4 · Scleraxis · SIM (1, 2) · TAL (1, 2) · Twist · USF1(1.4) NF-1(1.5) RF-X(1.6) Basic helix-span-helix (bHSH)(2) Zinc finger DNA-binding domains subfamily 1 (Thyroid hormone (α, β), CAR, FXR, LXR (α, β), PPAR (α, β/δ, γ), PXR, RAR (α, β, γ), ROR (α, β, γ), Rev-ErbA (α, β), VDR)
subfamily 2 (COUP-TF (I, II), Ear-2, HNF4 (α, γ), PNR, RXR (α, β, γ), Testicular receptor (2, 4), TLX)
subfamily 3 (Steroid hormone (Androgen, Estrogen (α, β), Glucocorticoid, Mineralocorticoid, Progesterone), Estrogen related (α, β, γ))
subfamily 4 NUR (NGFIB, NOR1, NURR1) · subfamily 5 (LRH-1, SF1) · subfamily 6 (GCNF) · subfamily 0 (DAX1, SHP)(2.2) Other Cys4(2.3) Cys2His2General transcription factors (TFIIA, TFIIB, TFIID, TFIIE (1, 2), TFIIF (1, 2), TFIIH (1, 2, 4, 2I, 3A, 3C1, 3C2))
ATBF1 · BCL (6, 11A, 11B) · CTCF · E4F1 · EGR (1, 2, 3, 4) · ERV3 · GFI1 · GLI-Krüppel family (1, 2, 3, REST, S2, YY1) · HIC (1, 2) · HIVEP (1, 2, 3) · IKZF (1, 2, 3) · ILF (2, 3) · KLF (2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 17) · MTF1 · MYT1 · OSR1 · PRDM9 · SALL (1, 2, 3, 4) · SP (1, 2, 4, 7, 8) · TSHZ3 · WT1 · Zbtb7 (7A, 7B) · ZBTB (16, 17, 20, 32, 33, 40) · zinc finger (3, 7, 9, 10, 19, 22, 24, 33B, 34, 35, 41, 43, 44, 51, 74, 143, 146, 148, 165, 202, 217, 219, 238, 239, 259, 267, 268, 281, 295, 300, 318, 330, 346, 350, 365, 366, 384, 423, 451, 452, 471, 593, 638, 644, 649, 655)(2.4) Cys6(2.5) Alternating composition(3) Helix-turn-helix domains ARX · CDX (1, 2) · CRX · CUTL1 · DBX (1, 2) · DLX (3, 4, 5) · EMX2 · EN (1, 2) · FHL (1, 2, 3) · HESX1 · HHEX · HLX · Homeobox (A1, A2, A3, A4, A5, A7, A9, A10, A11, A13, B1, B2, B3, B4, B5, B6, B7, B8, B9, B13, C4, C5, C6, C8, C9, C10, C11, C12, C13, D1, D3, D4, D8, D9, D10, D11, D12, D13) · HOPX · IRX (1, 2, 3, 4, 5, 6, MKX) · LMX (1A, 1B) · MEIS (1, 2) · MEOX2 · MNX1 · MSX (1, 2) · NANOG · NKX (2-1, 2-2, 2-3, 2-5, 3-1, 3-2, 6-1, 6-2) · NOBOX · PBX (1, 2, 3) · PHF (1, 3, 6, 8, 10, 16, 17, 20, 21A) · PHOX (2A, 2B) · PITX (1, 2, 3) · POU domain (PIT-1, BRN-3: A, B, C, Octamer transcription factor: 1, 2, 3/4, 6, 7, 11) · OTX (1, 2) · PDX1 · SATB2 · SHOX2 · VAX1 · ZEB (1, 2)(3.2) Paired box(3.5) Tryptophan clusters(3.6) TEA domain(4) β-Scaffold factors with minor groove contacts (4.1) Rel homology region(4.3) p53(4.7) High-mobility group(4.10) Cold-shock domainCSDA, YBX1(4.11) Runt(0) Other transcription factors (0.2) HMGI(Y)(0.6) MiscellaneousCategories:- Human proteins
- Intracellular receptors
- Transcription factors
Wikimedia Foundation. 2010.
Look at other dictionaries:
Rev-ErbA — The Rev ErbA proteins are members of the nuclear receptor family of intracellular transcription factors. There are two forms of the receptor, alpha and beta, each encoded by a separate gene (gene|NR1D1 and gene|NR1D2 respectively).cite journal |… … Wikipedia
Rev-ErbA beta — Nuclear receptor subfamily 1, group D, member 2 Structure of the ligand binding domain (LBD) of the Rev erb? receptor based on PDB 2v0v. Depicted here is a dimer of comprising two LBD protein molecules … Wikipedia
Thyroid hormone receptor alpha — Thyroid hormone receptor, alpha PDB rendering based on 1nav … Wikipedia
Estrogen receptor alpha — Estrogen receptor 1 PDB rendering based on 1a52 … Wikipedia
RAR-related orphan receptor alpha — RAR related orphan receptor A PDB rendering based on 1n83 … Wikipedia
Retinoic acid receptor alpha — Retinoic acid receptor, alpha PDB rendering based on 1dkf … Wikipedia
Peroxisome proliferator-activated receptor alpha — PDB rendering based on 1i7g … Wikipedia
Retinoid X receptor alpha — Retinoid X receptor, alpha PDB rendering based on 1by4 … Wikipedia
Liver X receptor alpha — Nuclear receptor subfamily 1, group H, member 3 PDB rendering based on 1uhl … Wikipedia
Estrogen-related receptor alpha — PDB rendering based on 1lo1 … Wikipedia
Share the article and excerpts
Direct link
https://en-academic.com/dic.nsf/enwiki/7504481 Do a right-click on the link above
and select “Copy Link”
Rev-ErbA alpha
- Rev-ErbA alpha
-