- MAF (gene)
-
V-maf musculoaponeurotic fibrosarcoma oncogene homolog (avian) Identifiers Symbols MAF; MGC71685; c-MAF External IDs OMIM: 177075 MGI: 96909 HomoloGene: 74552 GeneCards: MAF Gene Gene Ontology Molecular function • sequence-specific DNA binding transcription factor activity
• protein binding
• sequence-specific DNA bindingCellular component • chromatin
• nucleusBiological process • transcription from RNA polymerase II promoter Sources: Amigo / QuickGO RNA expression pattern 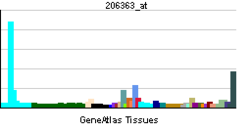
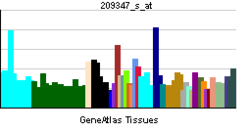
More reference expression data Orthologs Species Human Mouse Entrez 4094 17132 Ensembl ENSG00000178573 ENSMUSG00000055435 UniProt O75444 Q3V1Z2 RefSeq (mRNA) NM_001031804.2 NM_001025577.2 RefSeq (protein) NP_001026974.1 NP_001020748.2 Location (UCSC) Chr 16:
79.63 – 79.63 MbChr 8:
118.21 – 118.23 MbPubMed search [1] [2] Transcription factor Maf also known as proto-oncogene c-Maf or V-maf musculoaponeurotic fibrosarcoma oncogene homolog is a transcription factor that in humans is encoded by the MAF gene.[1][2]
Contents
Types
One type, MafA, also known as RIPE3b1,[3] promotes pancreatic development, as well as insulin gene transcription.[4]
Interactions
MAF has been shown to interact with:
References
- ^ "Entrez Gene: MAF v-maf musculoaponeurotic fibrosarcoma oncogene homolog (avian)". http://www.ncbi.nlm.nih.gov/sites/entrez?Db=gene&Cmd=ShowDetailView&TermToSearch=4094.
- ^ Nishizawa M, Kataoka K, Goto N, Fujiwara KT, Kawai S (October 1989). "v-maf, a viral oncogene that encodes a "leucine zipper" motif". Proc. Natl. Acad. Sci. U.S.A. 86 (20): 7711–5. doi:10.1073/pnas.86.20.7711. PMC 298140. PMID 2554284. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=298140.
- ^ Olbrot M, Rud J, Moss LG, Sharma A (May 2002). "Identification of β-cell-specific insulin gene transcription factor RIPE3b1 as mammalian MafA". Proc. Natl. Acad. Sci. U.S.A. 99 (10): 6737–42. doi:10.1073/pnas.102168499. PMC 124472. PMID 12011435. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=124472.
- ^ Melloul D, Marshak S, Cerasi E (March 2002). "Regulation of insulin gene transcription". Diabetologia 45 (3): 309–26. doi:10.1007/s00125-001-0728-y. PMID 11914736.
- ^ a b Chen Q, Dowhan DH, Liang D, Moore DD, Overbeek PA (July 2002). "CREB-binding protein/p300 co-activation of crystallin gene expression". J. Biol. Chem. 277 (27): 24081–9. doi:10.1074/jbc.M201821200. PMID 11943779.
- ^ Hedge SP, Kumar A, Kurschner C, Shapiro LH (May 1998). "c-Maf Interacts with c-Myb To Regulate Transcription of an Early Myeloid Gene during Differentiation". Mol. Cell. Biol. 18 (5): 2729–37. PMC 110652. PMID 9566892. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=110652.
- ^ Huang W, Lu N, Eberspaecher H, De Crombrugghe B (December 2002). "A new long form of c-Maf cooperates with Sox9 to activate the type II collagen gene". J. Biol. Chem. 277 (52): 50668–75. doi:10.1074/jbc.M206544200. PMID 12381733.
Further reading
- Kerppola TK, Curran T (1994). "Maf and Nrl can bind to AP-1 sites and form heterodimers with Fos and Jun". Oncogene 9 (3): 675–84. PMID 8108109.
- Kurschner C, Morgan JI (1997). "USF2/FIP associates with the b-Zip transcription factor, c-Maf, via its bHLH domain and inhibits c-Maf DNA binding activity". Biochem. Biophys. Res. Commun. 231 (2): 333–9. doi:10.1006/bbrc.1997.6097. PMID 9070273.
- Hedge SP, Kumar A, Kurschner C, Shapiro LH (1998). "c-Maf Interacts with c-Myb To Regulate Transcription of an Early Myeloid Gene during Differentiation". Mol. Cell. Biol. 18 (5): 2729–37. PMC 110652. PMID 9566892. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=110652.
- Chesi M, Bergsagel PL, Shonukan OO et al. (1998). "Frequent dysregulation of the c-maf proto-oncogene at 16q23 by translocation to an Ig locus in multiple myeloma". Blood 91 (12): 4457–63. PMID 9616139.
- Christodoulopoulos P, Cameron L, Nakamura Y et al. (2001). "TH2 cytokine-associated transcription factors in atopic and nonatopic asthma: evidence for differential signal transducer and activator of transcription 6 expression". J. Allergy Clin. Immunol. 107 (4): 586–91. doi:10.1067/mai.2001.114883. PMID 11295643.
- Jamieson RV, Perveen R, Kerr B et al. (2002). "Domain disruption and mutation of the bZIP transcription factor, MAF, associated with cataract, ocular anterior segment dysgenesis and coloboma". Hum. Mol. Genet. 11 (1): 33–42. doi:10.1093/hmg/11.1.33. PMID 11772997.
- Chen Q, Dowhan DH, Liang D et al. (2002). "CREB-binding protein/p300 co-activation of crystallin gene expression". J. Biol. Chem. 277 (27): 24081–9. doi:10.1074/jbc.M201821200. PMID 11943779.
- Dhakshinamoorthy S, Jaiswal AK (2002). "c-Maf negatively regulates ARE-mediated detoxifying enzyme genes expression and anti-oxidant induction". Oncogene 21 (34): 5301–12. doi:10.1038/sj.onc.1205642. PMID 12149651.
- Cousins DJ, Lee TH, Staynov DZ (2002). "Cytokine coexpression during human Th1/Th2 cell differentiation: direct evidence for coordinated expression of Th2 cytokines". J. Immunol. 169 (5): 2498–506. PMID 12193719.
- Huang W, Lu N, Eberspaecher H, De Crombrugghe B (2003). "A new long form of c-Maf cooperates with Sox9 to activate the type II collagen gene". J. Biol. Chem. 277 (52): 50668–75. doi:10.1074/jbc.M206544200. PMID 12381733.
- Strausberg RL, Feingold EA, Grouse LH et al. (2003). "Generation and initial analysis of more than 15,000 full-length human and mouse cDNA sequences". Proc. Natl. Acad. Sci. U.S.A. 99 (26): 16899–903. doi:10.1073/pnas.242603899. PMC 139241. PMID 12477932. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=139241.
- DePianto DJ, Blankenship TN, Hess JF, FitzGerald PG (2003). "Analysis of non-crystallin lens fiber cell gene expression in c-Maf -/- mice". Mol. Vis. 9: 288–94. PMID 12835653.
- Grimbert P, Valanciute A, Audard V et al. (2003). "Truncation of C-mip (Tc-mip), a New Proximal Signaling Protein, Induces c-maf Th2 Transcription Factor and Cytoskeleton Reorganization". J. Exp. Med. 198 (5): 797–807. doi:10.1084/jem.20030566. PMC 1865475. PMID 12939343. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=1865475.
- Valanciuté A, le Gouvello S, Solhonne B et al. (2004). "NF-kappa B p65 antagonizes IL-4 induction by c-maf in minimal change nephrotic syndrome". J. Immunol. 172 (1): 688–98. PMID 14688382.
- Rasmussen T, Knudsen LM, Dahl IM, Johnsen HE (2004). "C-MAF oncogene dysregulation in multiple myeloma: frequency and biological relevance". Leuk. Lymphoma 44 (10): 1761–6. doi:10.1080/1042819031000111035. PMID 14692531.
- Hurt EM, Wiestner A, Rosenwald A et al. (2004). "Overexpression of c-maf is a frequent oncogenic event in multiple myeloma that promotes proliferation and pathological interactions with bone marrow stroma". Cancer Cell 5 (2): 191–9. doi:10.1016/S1535-6108(04)00019-4. PMID 14998494.
- Cao S, Liu J, Song L, Ma X (2005). "The Protooncogene c-Maf Is an Essential Transcription Factor for IL-10 Gene Expression in Macrophages". J. Immunol. 174 (6): 3484–92. PMC 2955976. PMID 15749884. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2955976.
- Morito N, Yoh K, Fujioka Y et al. (2006). "Overexpression of c-Maf contributes to T-cell lymphoma in both mice and human". Cancer Res. 66 (2): 812–9. doi:10.1158/0008-5472.CAN-05-2154. PMID 16424013.
- Vanita V, Singh D, Robinson PN et al. (2006). "A novel mutation in the DNA-binding domain of MAF at 16q23.1 associated with autosomal dominant "cerulean cataract" in an Indian family". Am. J. Med. Genet. A 140 (6): 558–66. doi:10.1002/ajmg.a.31126. PMID 16470690.
External links
This article incorporates text from the United States National Library of Medicine, which is in the public domain.
Transcription factors and intracellular receptors (1) Basic domains (1.1) Basic leucine zipper (bZIP)Activating transcription factor (AATF, 1, 2, 3, 4, 5, 6, 7) · AP-1 (c-Fos, FOSB, FOSL1, FOSL2, JDP2, c-Jun, JUNB, JUND) · BACH (1, 2) · BATF · BLZF1 · C/EBP (α, β, γ, δ, ε, ζ) · CREB (1, 3, L1) · CREM · DBP · DDIT3 · GABPA · HLF · MAF (B, F, G, K) · NFE (2, L1, L2, L3) · NFIL3 · NRL · NRF (1, 2, 3) · XBP1(1.2) Basic helix-loop-helix (bHLH)ATOH1 · AhR · AHRR · ARNT · ASCL1 · BHLHB2 · BMAL (ARNTL, ARNTL2) · CLOCK · EPAS1 · FIGLA · HAND (1, 2) · HES (5, 6) · HEY (1, 2, L) · HES1 · HIF (1A, 3A) · ID (1, 2, 3, 4) · LYL1 · MESP2 · MXD4 · MYCL1 · MYCN · Myogenic regulatory factors (MyoD, Myogenin, MYF5, MYF6) · Neurogenins (1, 2, 3) · NeuroD (1, 2) · NPAS (1, 2, 3) · OLIG (1, 2) · Pho4 · Scleraxis · SIM (1, 2) · TAL (1, 2) · Twist · USF1(1.3) bHLH-ZIP(1.4) NF-1(1.5) RF-X(1.6) Basic helix-span-helix (bHSH)(2) Zinc finger DNA-binding domains (2.1) Nuclear receptor (Cys4)subfamily 1 (Thyroid hormone (α, β), CAR, FXR, LXR (α, β), PPAR (α, β/δ, γ), PXR, RAR (α, β, γ), ROR (α, β, γ), Rev-ErbA (α, β), VDR)
subfamily 2 (COUP-TF (I, II), Ear-2, HNF4 (α, γ), PNR, RXR (α, β, γ), Testicular receptor (2, 4), TLX)
subfamily 3 (Steroid hormone (Androgen, Estrogen (α, β), Glucocorticoid, Mineralocorticoid, Progesterone), Estrogen related (α, β, γ))
subfamily 4 NUR (NGFIB, NOR1, NURR1) · subfamily 5 (LRH-1, SF1) · subfamily 6 (GCNF) · subfamily 0 (DAX1, SHP)(2.2) Other Cys4(2.3) Cys2His2General transcription factors (TFIIA, TFIIB, TFIID, TFIIE (1, 2), TFIIF (1, 2), TFIIH (1, 2, 4, 2I, 3A, 3C1, 3C2))
ATBF1 · BCL (6, 11A, 11B) · CTCF · E4F1 · EGR (1, 2, 3, 4) · ERV3 · GFI1 · GLI-Krüppel family (1, 2, 3, REST, S2, YY1) · HIC (1, 2) · HIVEP (1, 2, 3) · IKZF (1, 2, 3) · ILF (2, 3) · KLF (2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 17) · MTF1 · MYT1 · OSR1 · PRDM9 · SALL (1, 2, 3, 4) · SP (1, 2, 4, 7, 8) · TSHZ3 · WT1 · Zbtb7 (7A, 7B) · ZBTB (16, 17, 20, 32, 33, 40) · zinc finger (3, 7, 9, 10, 19, 22, 24, 33B, 34, 35, 41, 43, 44, 51, 74, 143, 146, 148, 165, 202, 217, 219, 238, 239, 259, 267, 268, 281, 295, 300, 318, 330, 346, 350, 365, 366, 384, 423, 451, 452, 471, 593, 638, 644, 649, 655)(2.4) Cys6(2.5) Alternating composition(3) Helix-turn-helix domains (3.1) HomeodomainARX · CDX (1, 2) · CRX · CUTL1 · DBX (1, 2) · DLX (3, 4, 5) · EMX2 · EN (1, 2) · FHL (1, 2, 3) · HESX1 · HHEX · HLX · Homeobox (A1, A2, A3, A4, A5, A7, A9, A10, A11, A13, B1, B2, B3, B4, B5, B6, B7, B8, B9, B13, C4, C5, C6, C8, C9, C10, C11, C13, D1, D3, D4, D8, D9, D10, D11, D12, D13) · HOPX · IRX (1, 2, 3, 4, 5, 6, MKX) · LMX (1A, 1B) · MEIS (1, 2) · MEOX2 · MNX1 · MSX (1, 2) · NANOG · NKX (2-1, 2-2, 2-3, 2-5, 3-1, 3-2, 6-1, 6-2) · NOBOX · PBX (1, 2, 3) · PHF (1, 3, 6, 8, 10, 16, 17, 20, 21A) · PHOX (2A, 2B) · PITX (1, 2, 3) · POU domain (PIT-1, BRN-3: A, B, C, Octamer transcription factor: 1, 2, 3/4, 6, 7, 11) · OTX (1, 2) · PDX1 · SATB2 · SHOX2 · VAX1 · ZEB (1, 2)(3.2) Paired box(3.3) Fork head / winged helix(3.4) Heat Shock Factors(3.5) Tryptophan clusters(3.6) TEA domain(4) β-Scaffold factors with minor groove contacts (4.1) Rel homology region(4.2) STAT(4.3) p53(4.4) MADS box(4.6) TATA binding proteins(4.7) High-mobility group(4.10) Cold-shock domainCSDA, YBX1(4.11) Runt(0) Other transcription factors (0.2) HMGI(Y)(0.3) Pocket domain(0.6) Miscellaneoussee also transcription factor/coregulator deficiencies
B bsyn: dna (repl, cycl, reco, repr) · tscr (fact, tcrg, nucl, rnat, rept, ptts) · tltn (risu, pttl, nexn) · dnab, rnab/runp · stru (domn, 1°, 2°, 3°, 4°)Categories:- Human proteins
- Chromosome 16 gene stubs
- Transcription factors
Wikimedia Foundation. 2010.
