- Retinoid X receptor beta
-
Retinoid X receptor beta (RXR-beta), also known as NR2B2 (nuclear receptor subfamily 2, group B, member 2) is a nuclear receptor that in humans is encoded by the RXRB gene.[1][2]
This gene encodes a member of the retinoid X receptor (RXR) family of nuclear receptors which are involved in mediating the effects of retinoic acid (RA). This receptor forms heterodimers with the retinoic acid, thyroid hormone, and vitamin D receptors, increasing both DNA binding and transcriptional function on their respective response elements. The gene lies within the major histocompatibility complex (MHC) class II region on chromosome 6. An alternatively spliced transcript variant has been described, but its full length sequence has not been determined.[2]
See also
References
- ^ Fitzgibbon J, Gillett GT, Woodward KJ, Boyle JM, Wolfe J, Povey S (July 1993). "Mapping of RXRB to human chromosome 6p21.3". Ann. Hum. Genet. 57 (Pt 3): 203–9. doi:10.1111/j.1469-1809.1993.tb01596.x. PMID 8257090.
- ^ a b "Entrez Gene: RXRB retinoid X receptor, beta". http://www.ncbi.nlm.nih.gov/sites/entrez?Db=gene&Cmd=ShowDetailView&TermToSearch=6257.
Further reading
- Szanto A, Narkar V, Shen Q, et al. (2005). "Retinoid X receptors: X-ploring their (patho)physiological functions.". Cell Death Differ. 11 Suppl 2: S126–43. doi:10.1038/sj.cdd.4401533. PMID 15608692.
- Leid M, Kastner P, Lyons R, et al. (1992). "Purification, cloning, and RXR identity of the HeLa cell factor with which RAR or TR heterodimerizes to bind target sequences efficiently.". Cell 68 (2): 377–95. doi:10.1016/0092-8674(92)90478-U. PMID 1310259.
- Fleischhauer K, Park JH, DiSanto JP, et al. (1992). "Isolation of a full-length cDNA clone encoding a N-terminally variant form of the human retinoid X receptor beta". Nucleic Acids Res. 20 (7): 1801. doi:10.1093/nar/20.7.1801. PMC 312273. PMID 1315958. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=312273.
- Berrodin TJ, Marks MS, Ozato K, et al. (1992). "Heterodimerization among thyroid hormone receptor, retinoic acid receptor, retinoid X receptor, chicken ovalbumin upstream promoter transcription factor, and an endogenous liver protein". Mol. Endocrinol. 6 (9): 1468–78. doi:10.1210/me.6.9.1468. PMID 1331778.
- Epplen C, Epplen JT (1992). "The human cDNA sequence homologous to the mouse MHC class I promoter-binding protein gene contains four additional codons in lymphocytes". Mamm. Genome 3 (8): 472–5. doi:10.1007/BF00356161. PMID 1514958.
- Yu VC, Delsert C, Andersen B, et al. (1992). "RXR beta: a coregulator that enhances binding of retinoic acid, thyroid hormone, and vitamin D receptors to their cognate response elements". Cell 67 (6): 1251–66. doi:10.1016/0092-8674(91)90301-E. PMID 1662118.
- Oñate SA, Tsai SY, Tsai MJ, O'Malley BW (1995). "Sequence and characterization of a coactivator for the steroid hormone receptor superfamily". Science 270 (5240): 1354–7. doi:10.1126/science.270.5240.1354. PMID 7481822.
- Kooistra T, Lansink M, Arts J, et al. (1995). "Involvement of retinoic acid receptor alpha in the stimulation of tissue-type plasminogen-activator gene expression in human endothelial cells". Eur. J. Biochem. 232 (2): 425–32. doi:10.1111/j.1432-1033.1995.425zz.x. PMID 7556191.
- Perlmann T, Jansson L (1995). "A novel pathway for vitamin A signaling mediated by RXR heterodimerization with NGFI-B and NURR1". Genes Dev. 9 (7): 769–82. doi:10.1101/gad.9.7.769. PMID 7705655.
- Forman BM, Umesono K, Chen J, Evans RM (1995). "Unique response pathways are established by allosteric interactions among nuclear hormone receptors". Cell 81 (4): 541–50. doi:10.1016/0092-8674(95)90075-6. PMID 7758108.
- Zanaria E, Muscatelli F, Bardoni B, et al. (1995). "An unusual member of the nuclear hormone receptor superfamily responsible for X-linked adrenal hypoplasia congenita". Nature 372 (6507): 635–41. doi:10.1038/372635a0. PMID 7990953.
- Almasan A, Mangelsdorf DJ, Ong ES, et al. (1994). "Chromosomal localization of the human retinoid X receptors". Genomics 20 (3): 397–403. doi:10.1006/geno.1994.1193. PMID 8034312.
- Maruyama K, Sugano S (1994). "Oligo-capping: a simple method to replace the cap structure of eukaryotic mRNAs with oligoribonucleotides". Gene 138 (1–2): 171–4. doi:10.1016/0378-1119(94)90802-8. PMID 8125298.
- Fleischhauer K, McBride OW, DiSanto JP, et al. (1993). "Cloning and chromosome mapping of human retinoid X receptor beta: selective amino acid sequence conservation of a nuclear hormone receptor in mammals". Hum. Genet. 90 (5): 505–10. doi:10.1007/BF00217449. PMID 8381386.
- May M, Mengus G, Lavigne AC, et al. (1996). "Human TAF(II28) promotes transcriptional stimulation by activation function 2 of the retinoid X receptors". EMBO J. 15 (12): 3093–104. PMC 450252. PMID 8670810. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=450252.
- Bonaldo MF, Lennon G, Soares MB (1997). "Normalization and subtraction: two approaches to facilitate gene discovery". Genome Res. 6 (9): 791–806. doi:10.1101/gr.6.9.791. PMID 8889548.
- Joyeux A, Cavaillès V, Balaguer P, Nicolas JC (1997). "RIP 140 enhances nuclear receptor-dependent transcription in vivo in yeast". Mol. Endocrinol. 11 (2): 193–202. doi:10.1210/me.11.2.193. PMID 9013766.
- Xu XC, Sozzi G, Lee JS, et al. (1997). "Suppression of retinoic acid receptor beta in non-small-cell lung cancer in vivo: implications for lung cancer development". J. Natl. Cancer Inst. 89 (9): 624–9. doi:10.1093/jnci/89.9.624. PMID 9150186.
- Chen H, Lin RJ, Schiltz RL, et al. (1997). "Nuclear receptor coactivator ACTR is a novel histone acetyltransferase and forms a multimeric activation complex with P/CAF and CBP/p300". Cell 90 (3): 569–80. doi:10.1016/S0092-8674(00)80516-4. PMID 9267036.
This article incorporates text from the United States National Library of Medicine, which is in the public domain.
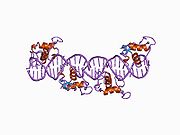
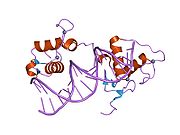
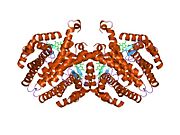
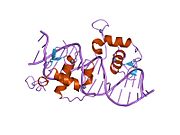
PDB gallery 1by4: STRUCTURE AND MECHANISM OF THE HOMODIMERIC ASSEMBLY OF THE RXR ON DNA1dsz: STRUCTURE OF THE RXR/RAR DNA-BINDING DOMAIN HETERODIMER IN COMPLEX WITH THE RETINOIC ACID RESPONSE ELEMENT DR11h9u: THE STRUCTURE OF THE HUMAN RETINOID-X-RECEPTOR BETA LIGAND BINDING DOMAIN IN COMPLEX WITH THE SPECIFIC SYNTHETIC AGONIST LG1002681r0n: Crystal Structure of Heterodimeric Ecdsyone receptor DNA binding complex1rxr: HIGH RESOLUTION SOLUTION STRUCTURE OF THE RETINOID X RECEPTOR DNA BINDING DOMAIN, NMR, 20 STRUCTURE1uhl: Crystal structure of the LXRalfa-RXRbeta LBD heterodimer2nll: RETINOID X RECEPTOR-THYROID HORMONE RECEPTOR DNA-BINDING DOMAIN HETERODIMER BOUND TO THYROID RESPONSE ELEMENT DNATranscription factors and intracellular receptors (1) Basic domains (1.1) Basic leucine zipper (bZIP)Activating transcription factor (AATF, 1, 2, 3, 4, 5, 6, 7) · AP-1 (c-Fos, FOSB, FOSL1, FOSL2, JDP2, c-Jun, JUNB, JUND) · BACH (1, 2) · BATF · BLZF1 · C/EBP (α, β, γ, δ, ε, ζ) · CREB (1, 3, L1) · CREM · DBP · DDIT3 · GABPA · HLF · MAF (B, F, G, K) · NFE (2, L1, L2, L3) · NFIL3 · NRL · NRF (1, 2, 3) · XBP1(1.2) Basic helix-loop-helix (bHLH)ATOH1 · AhR · AHRR · ARNT · ASCL1 · BHLHB2 · BMAL (ARNTL, ARNTL2) · CLOCK · EPAS1 · FIGLA · HAND (1, 2) · HES (5, 6) · HEY (1, 2, L) · HES1 · HIF (1A, 3A) · ID (1, 2, 3, 4) · LYL1 · MESP2 · MXD4 · MYCL1 · MYCN · Myogenic regulatory factors (MyoD, Myogenin, MYF5, MYF6) · Neurogenins (1, 2, 3) · NeuroD (1, 2) · NPAS (1, 2, 3) · OLIG (1, 2) · Pho4 · Scleraxis · SIM (1, 2) · TAL (1, 2) · Twist · USF1(1.3) bHLH-ZIP(1.4) NF-1(1.5) RF-X(1.6) Basic helix-span-helix (bHSH)(2) Zinc finger DNA-binding domains (2.1) Nuclear receptor (Cys4)subfamily 1 (Thyroid hormone (α, β), CAR, FXR, LXR (α, β), PPAR (α, β/δ, γ), PXR, RAR (α, β, γ), ROR (α, β, γ), Rev-ErbA (α, β), VDR)
subfamily 2 (COUP-TF (I, II), Ear-2, HNF4 (α, γ), PNR, RXR (α, β, γ), Testicular receptor (2, 4), TLX)
subfamily 3 (Steroid hormone (Androgen, Estrogen (α, β), Glucocorticoid, Mineralocorticoid, Progesterone), Estrogen related (α, β, γ))
subfamily 4 NUR (NGFIB, NOR1, NURR1) · subfamily 5 (LRH-1, SF1) · subfamily 6 (GCNF) · subfamily 0 (DAX1, SHP)(2.2) Other Cys4(2.3) Cys2His2General transcription factors (TFIIA, TFIIB, TFIID, TFIIE (1, 2), TFIIF (1, 2), TFIIH (1, 2, 4, 2I, 3A, 3C1, 3C2))
ATBF1 · BCL (6, 11A, 11B) · CTCF · E4F1 · EGR (1, 2, 3, 4) · ERV3 · GFI1 · GLI-Krüppel family (1, 2, 3, REST, S2, YY1) · HIC (1, 2) · HIVEP (1, 2, 3) · IKZF (1, 2, 3) · ILF (2, 3) · KLF (2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 17) · MTF1 · MYT1 · OSR1 · PRDM9 · SALL (1, 2, 3, 4) · SP (1, 2, 4, 7, 8) · TSHZ3 · WT1 · Zbtb7 (7A, 7B) · ZBTB (16, 17, 20, 32, 33, 40) · zinc finger (3, 7, 9, 10, 19, 22, 24, 33B, 34, 35, 41, 43, 44, 51, 74, 143, 146, 148, 165, 202, 217, 219, 238, 239, 259, 267, 268, 281, 295, 300, 318, 330, 346, 350, 365, 366, 384, 423, 451, 452, 471, 593, 638, 644, 649, 655)(2.4) Cys6(2.5) Alternating composition(3) Helix-turn-helix domains (3.1) HomeodomainARX · CDX (1, 2) · CRX · CUTL1 · DBX (1, 2) · DLX (3, 4, 5) · EMX2 · EN (1, 2) · FHL (1, 2, 3) · HESX1 · HHEX · HLX · Homeobox (A1, A2, A3, A4, A5, A7, A9, A10, A11, A13, B1, B2, B3, B4, B5, B6, B7, B8, B9, B13, C4, C5, C6, C8, C9, C10, C11, C12, C13, D1, D3, D4, D8, D9, D10, D11, D12, D13) · HOPX · IRX (1, 2, 3, 4, 5, 6, MKX) · LMX (1A, 1B) · MEIS (1, 2) · MEOX2 · MNX1 · MSX (1, 2) · NANOG · NKX (2-1, 2-2, 2-3, 2-5, 3-1, 3-2, 6-1, 6-2) · NOBOX · PBX (1, 2, 3) · PHF (1, 3, 6, 8, 10, 16, 17, 20, 21A) · PHOX (2A, 2B) · PITX (1, 2, 3) · POU domain (PIT-1, BRN-3: A, B, C, Octamer transcription factor: 1, 2, 3/4, 6, 7, 11) · OTX (1, 2) · PDX1 · SATB2 · SHOX2 · VAX1 · ZEB (1, 2)(3.2) Paired box(3.3) Fork head / winged helix(3.4) Heat Shock Factors(3.5) Tryptophan clusters(3.6) TEA domain(4) β-Scaffold factors with minor groove contacts (4.1) Rel homology region(4.2) STAT(4.3) p53(4.4) MADS box(4.6) TATA binding proteins(4.7) High-mobility group(4.10) Cold-shock domainCSDA, YBX1(4.11) Runt(0) Other transcription factors (0.2) HMGI(Y)(0.3) Pocket domain(0.6) MiscellaneousCategories:- Human proteins
- Chromosome 6 gene stubs
- Intracellular receptors
- Transcription factors
Wikimedia Foundation. 2010.