- Alternative splicing
-
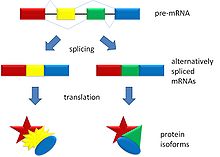
Alternative splicing (or differential splicing) is a process by which the exons of the RNA produced by transcription of a gene (a primary gene transcript or pre-mRNA) are reconnected in multiple ways during RNA splicing. The resulting different mRNAs may be translated into different protein isoforms; thus, a single gene may code for multiple proteins.[1]
Alternative splicing occurs as a normal phenomenon in eukaryotes, where it greatly increases the diversity of proteins that can be encoded by the genome;[1] in humans, ~95% of multiexonic genes are alternatively spliced.[2] There are numerous modes of alternative splicing observed, of which the most common is exon skipping. In this mode, a particular exon may be included in mRNAs under some conditions or in particular tissues, and omitted from the mRNA in others.[1]
The production of alternatively spliced mRNAs is regulated by a system of trans-acting proteins that bind to cis-acting sites on the pre-mRNA itself. Such proteins include splicing activators that promote the usage of a particular splice site, and splicing repressors that reduce the usage of a particular site. Mechanisms of alternative splicing are highly variable, and new examples are constantly being found, particularly through the use of high-throughput techniques. Researchers hope to fully elucidate the regulatory systems involved in splicing, so that alternative splicing products from a given gene under particular conditions could be predicted by a "splicing code".[3][4]
Abnormal variations in splicing are also implicated in disease; a large proportion of human genetic disorders result from splicing variants.[3] Abnormal splicing variants are also thought to contribute to the development of cancer,[5][6][7] although such aberrant splicing products are, under normal conditions, usually safeguarded and eliminated by a posttranscriptional quality control mechanism.[8]
Contents
Discovery
Alternative splicing was first observed in 1977.[9][10] Adenoviruses produce two different primary transcripts, one early in the life cycle and one later, after DNA replication. Researchers found that the primary RNA transcript produced by adenovirus type 2 in the late phase was spliced in different ways, resulting in mRNAs encoding different viral proteins. Both 5’ and 3’ splice sites varied, and in addition, the transcript contained multiple polyadenylation sites, giving different 3’ ends for the processed mRNAs.[11][12][13]
In 1981, the first example of alternative splicing in a transcript from a normal, endogenous gene was characterized.[11] The gene encoding the thyroid hormone calcitonin was found to be alternatively spliced in mammalian cells. The pre-mRNA from this gene contains 6 exons; the calcitonin mRNA contains exons 1–4, and terminates after a polyadenylation site in exon 4. Another mRNA is produced from this pre-mRNA by skipping exon 4, and includes exons 1–3, 5, and 6. It encodes a protein known as CGRP (calcitonin gene related peptide).[14][15] Examples of alternative splicing in immunoglobin gene transcripts in mammals were also observed in the early 1980s.[11][16]
Since then, alternative splicing has been found to be ubiquitous in eukaryotes.[1] The "record-holder" for alternative splicing is a D. melanogaster gene called Dscam, which could potentially have 38,016 splice variants.[17]
Modes
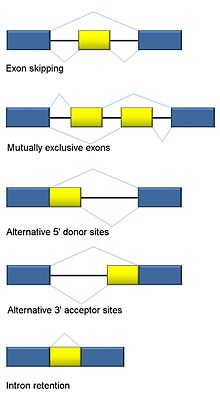
Five basic modes of alternative splicing are generally recognized.[1][2][3][18]
- Exon skipping or cassette exon: in this case, an exon may be spliced out of the primary transcript or retained. This is the most common mode in mammalian pre-mRNAs.[18]
- Mutually exclusive exons: One of two exons is retained in mRNAs after splicing, but not both.
- Alternative donor site: An alternative 5' splice junction (donor site) is used, changing the 3' boundary of the upstream exon.
- Alternative acceptor site: An alternative 3' splice junction (acceptor site) is used, changing the 5' boundary of the downstream exon.
- Intron retention: A sequence may be spliced out as an intron or simply retained. This is distinguished from exon skipping because the retained sequence is not flanked by introns. If the retained intron is in the coding region, the intron must encode amino acids in frame with the neighboring exons, or a stop codon or a shift in the reading frame will cause the protein to be non-functional. This is the rarest mode in mammals.[18]
In addition to these primary modes of alternative splicing, there are two other main mechanisms by which different mRNAs may be generated from the same gene; multiple promoters and multiple polyadenylation sites. Use of multiple promoters is properly described as a transcriptional regulation mechanism rather than alternative splicing; by starting transcription at different points, transcripts with different 5'-most exons can be generated. At the other end, multiple polyadenylation sites provide different 3' end points for the transcript. Both of these mechanisms are found in combination with alternative splicing and provide additional variety in mRNAs derived from a gene.[1][3]
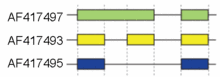 Schematic cutoff from 3 splicing structures in the murine hyaluronidase gene. Directionality of transcription from 5' to 3' is shown from left to right. Exons and introns are not drawn to scale.
Schematic cutoff from 3 splicing structures in the murine hyaluronidase gene. Directionality of transcription from 5' to 3' is shown from left to right. Exons and introns are not drawn to scale.
These modes describe basic splicing mechanisms, but may be inadequate to describe complex splicing events. For instance, the figure to the right shows 3 spliceforms from the mouse hyaluronidase 3 gene. Comparing the exonic structure shown in the first line (green) with the one in the second line (yellow) shows intron retention, whereas the comparison between the second and the third spliceform (yellow vs. blue) exhibits exon skipping. A model nomenclature to uniquely designate all possible splicing patterns has recently been proposed.[18]Alternative splicing mechanisms
General splicing mechanism
When the pre-mRNA has been transcribed from the DNA, it includes several introns and exons. (In nematodes, the mean is 4–5 exons and introns; in the fruit fly Drosophila there can be more than 100 introns and exons in one transcribed pre-mRNA.) The exons to be retained in the mRNA are determined during the splicing process. The regulation and selection of splice sites are done by trans-acting splicing activator and splicing repressor proteins.
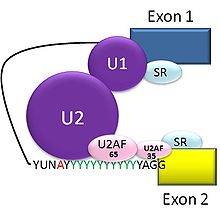
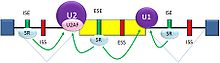
The typical eukaryotic nuclear intron has consensus sequences defining important regions. Each intron has GU at its 5' end. Near the 3' end there is a branch site. The nucleotide at the branch point is always an A; the consensus around this sequence varies somewhat. In humans the branch consensus is yUnAy.[19] The branch site is followed by a series of pyrimidines, or polypyrimidine tract, then by AG at 3' end.[3]
Splicing of mRNA is performed by an RNA and protein complex known as the spliceosome, containing snRNPs designated U1, U2, U4, U5, and U6 (U3 is not involved in mRNA splicing).[20] U1 binds to 5' GU and U2 binds to branch site (A) with the assistance of the U2AF protein factors. The complex at this stage is known as the spliceosome A complex. Formation of the A complex is usually the key step in determining the ends of the intron to be spliced out, and defining the ends of the exon to be retained.[3]
The U4,U5,U6 complex binds, and U6 replaces the U1 position. U1 and U4 leave. The remaining complex then performs two transesterification reactions. In the first transesterification, 5' end of the intron is cleaved from the upstream exon and joined to the branch site A by a 2',5'-phosphodiester linkage. In the second transesterification, the 3' end of the intron is cleaved from the downstream exon, and the two exons are joined by a phosphodiester bond. The intron is then released in lariat form and degraded.[1]
Regulatory elements and proteins
Splicing is regulated by trans-acting proteins (repressors and activators), corresponding cis-acting regulatory sites (silencers and enhancers) on the RNA, and other RNA features that influence how splicing will occur, such as secondary structures. Together, these elements form a "splicing code" that governs how splicing will occur under different cellular conditions [21] [22].
The determinants of splicing act in an inter-dependent fashion, but in many cases a particular determinant will have a consistent influence on splicing. Among these cases, there are two major types of cis-acting RNA sequence elements present in pre-mRNAs and they have corresponding trans-acting RNA-binding proteins. Splicing silencers are sites to which splicing repressor proteins bind, reducing the probability that a nearby site will be used as a splice junction. These can be located in the intron itself (intronic splicing silencers, ISS) or in a neighboring exon (exonic splicing silencers, ESS). They vary in sequence, as well as in the types of proteins that bind to them. The majority of splicing repressors are heterogeneous nuclear ribonucleoproteins (hnRNPs) such as hnRNPA1 and polypyrimidine tract binding protein (PTB).[3][21]
Splicing enhancers are sites to which splicing activator proteins bind, increasing the probability that a nearby site will be used as a splice junction. These also may occur in the intron (intronic splicing enhancers, ISE) or exon (exonic splicing enhancers, ESE). Most of the activator proteins that bind to ISEs and ESEs are members of the SR protein family. Such proteins contain RNA recognition motifs and arginine and serine-rich (RS) domains.[3][21]
The adaptive significance of splicing silencers and enhancers is attested by a study showing that there is strong selection in human genes against mutations that produce new silencers or disrupt existing enhancers.[23]
In general, the determinants of splicing work in an inter-dependent manner that depends on context, so that the rules governing how splicing is regulated form a splicing code.[22] The presence of a particular cis-acting RNA sequence element may increase the probability that a nearby site will be spliced in some cases, but decrease the probability in other cases, depending on context. The context within which regulatory elements act includes cis-acting context that is established by the presence of other RNA sequence features, and trans-acting context that is established by cellular conditions. For example, some cis-acting RNA sequence elements influence splicing only if multiple elements are present in the same region so as to establish context. As another example, a cis-acting element can have opposite effects on splicing, depending on which proteins are expressed in the cell (e.g., neuronal versus non-neuronal PTB).
Examples
Exon skipping: Drosophila dsx
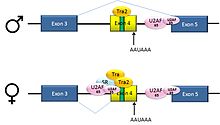
Pre-mRNAs from the D. melanogaster gene dsx contain 6 exons. In males, exons 1,2,3,5,and 6 are joined to form the mRNA, which encodes a transcriptional regulatory protein required for male development. In females, exons 1,2,3, and 4 are joined, and a polyadenylation signal in exon 4 causes cleavage of the mRNA at that point. The resulting mRNA is a transcriptional regulatory protein required for female development.[24]
This is an example of exon skipping. The intron upstream from exon 4 has a polypyrimidine tract that doesn't match the consensus sequence well, so that U2AF proteins bind poorly to it without assistance from splicing activators. This 3' splice acceptor site is therefore not used in males. Females, however, produce the splicing activator Transformer (Tra)(see below). The SR protein Tra2 is produced in both sexes and binds to an ESE in exon 4; if Tra is present, it binds to Tra2 and, along with another SR protein, forms a complex that assists U2AF proteins in binding to the weak polypyrimidine tract. U2 is recruited to the associated branch point, and this leads to inclusion of exon 4 in the mRNA.[24][25]
Alternative acceptor sites: Drosophila Transformer
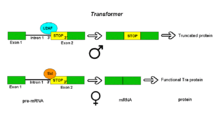
Pre-mRNAs of the Transformer (Tra) gene of Drosophila melanogaster undergo alternative splicing via the alternative acceptor site mode. The gene Tra encodes a protein that is expressed only in females. The primary transcript of this gene contains an intron with two possible acceptor sites. In males, the upstream acceptor site is used. This causes a longer version of exon 2 to be included in the processed transcript, including an early stop codon. The resulting mRNA encodes a truncated protein product that is inactive. Females produce the master sex determination protein Sex lethal (Sxl). The Sxl protein is a splicing repressor that binds to an ISS in the RNA of the Tra transcript near the upstream acceptor site, preventing U2AF protein from binding to the polypyrimidine tract. This prevents the use of this junction, shifting the spliceosome binding to the downstream acceptor site. Splicing at this point bypasses the stop codon, which is excised as part of the intron. The resulting mRNA encodes an active Tra protein, which itself is a regulator of alternative splicing of other sex-related genes (see dsx above).[1]
Exon definition: Fas receptor
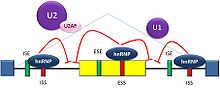
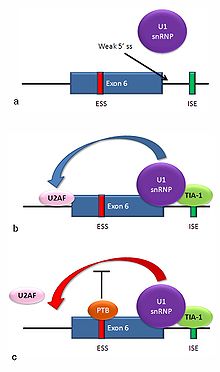
Multiple isoforms of the Fas receptor protein are produced by alternative splicing. Two normally occurring isoforms in humans are produced by an exon-skipping mechanism. An mRNA including exon 6 encodes the membrane-bound form of the Fas receptor, which promotes apoptosis, or programmed cell death. Increased expression of Fas receptor in skin cells chronically exposed to the sun, and absence of expression in skin cancer cells, suggests that this mechanism may be important in elimination of pre-cancerous cells in humans.[26] If exon 6 is skipped, the resulting mRNA encodes a soluble Fas protein that does not promote apoptosis. The inclusion or skipping of the exon depends on two antagonistic proteins, TIA-1 and polypyrimidine tract-binding protein (PTB).
- The 5' donor site in the intron downstream from exon 6 in the pre-mRNA has a weak agreement with the consensus sequence, and is not bound usually by the U1 snRNP. If U1 does not bind, the exon is skipped (see "a" in accompanying figure).
- Binding of TIA-1 protein to an intronic splicing enhancer site stabilizes binding of the U1 snRNP.[3] The resulting 5' donor site complex assists in binding of the splicing factor U2AF to the 3' splice site upstream of the exon, through a mechanism that is not yet known (see b).[27]
- Exon 6 contains a pyrimidine-rich exonic splicing silencer, ure6, where PTB can bind. If PTB binds, it inhibits the effect of the 5' donor complex on the binding of U2AF to the acceptor site, resulting in exon skipping (see c).
This mechanism is an example of exon definition in splicing. A spliceosome assembles on an intron, and the snRNP subunits bring fold the RNA so that the 5' and 3' ends of the intron are joined. However, recently studied examples such as this one show that there are also interactions between the ends of the exon. In this particular case, these exon definition interactions are necessary to allow the binding of core splicing factors prior to assembly of the spliceosomes on the two flanking introns.[27]
Repressor-activator competition: HIV-1 tat exon 2
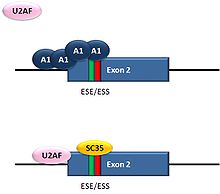
HIV, the retrovirus that causes AIDS in humans, produces a single primary RNA transcript, which is alternatively spliced in multiple ways to produce over 40 different mRNAs.[28] Equilibrium among differentially spliced transcripts provides multiple mRNAs encoding different products that are required for viral multiplication.[29] One of the differentially spliced transcripts contains the tat gene, in which exon 2 is a cassette exon that may be skipped or included. The inclusion of tat exon 2 in the RNA is regulated by competition between the splicing repressor hnRNP A1 and the SR protein SC35. Within exon 2 an exonic splicing silencer sequence (ESS) and an exonic splicing enhancer sequence (ESE) overlap. If A1 repressor protein binds to the ESS, it initiates cooperative binding of multiple A1 molecules, extending into the 5’ donor site upstream of exon 2 and preventing the binding of the core splicing factor U2AF35 to the polypyrimidine tract. If SC35 binds to the ESE, it prevents A1 binding and maintains the 5’ donor site in an accessible state for assembly of the spliceosome. Competition between the activator and repressor ensures that both mRNA types (with and without exon 2) are produced.[28]
Adaptive significance
Alternative splicing is one of several exceptions to the original idea that one DNA sequence codes for one polypeptide (the One gene-one enzyme hypothesis). It might be more correct now to say "One gene – many polypeptides.[30]" External information is needed in order to decide which polypeptide is produced, given a DNA sequence and pre-mRNA. Since the methods of regulation are inherited, this provides novel ways for mutations to affect gene expression.[7]
It has been proposed that for eukaryotes alternative splicing was a very important step towards higher efficiency, because information can be stored much more economically. Several proteins can be encoded by a single gene, rather than requiring a separate gene for each, and thus allowing a more varied proteome from a genome of limited size.[1] It also provides evolutionary flexibility. A single point mutation may cause a given exon to be occasionally excluded or included from a transcript during splicing, allowing production of a new protein isoform without loss of the original protein.[1] Comparative studies indicate that alternative splicing preceded multicellularity in evolution, and suggest that this mechanism might have been co-opted to assist in the development of multicellular organisms.[31]
Research based on the Human Genome Project and other genome sequencing has shown that humans have only about 30% more genes than the roundworm Caenorhabditis elegans, and only about twice as many as the fly Drosophila melanogaster. This finding led to speculation that the perceived greater complexity of humans, or vertebrates generally, might be due to higher rates of alternative splicing in humans than are found in invertebrates.[32][33] However, a study on samples of 100,000 ESTs each from human, mouse, rat, cow, fly (D. melanogaster), worm (C. elegans), and the plant Arabidopsis thaliana found no large differences in frequency of alternatively spliced genes among humans and any of the other animals tested.[34] Another study, however, proposed that these results were an artifact of the different numbers of ESTs available for the various organisms. When they compared alternative splicing frequencies in random subsets of genes from each organism, the authors concluded that vertebrates do have higher rates of alternative splicing than invertebrates.[35]
Alternative splicing and disease
Changes in the RNA processing machinery may lead to mis-splicing of multiple transcripts, while single-nucleotide alterations in splice sites or cis-acting splicing regulatory sites may lead to differences in splicing of a single gene, and thus in the mRNA produced from a mutant gene's transcripts. A probabilistic analysis indicates that over 60% of human disease-causing mutations affect splicing rather than directly affecting coding sequences.[36]
Abnormally spliced mRNAs are also found in a high proportion of cancerous cells.[5][6] Until recently, it was unclear whether such aberrant patterns of splicing played a role in causing cancerous growth, or were merely a consequence of cellular abnormalities associated with cancer. It has been shown that there is actually a reduction of alternative splicing in cancerous cells compared to normal ones, and the types of splicing differ; for instance, cancerous cells show higher levels of intron retention than normal cells, but lower levels of exon skipping.[37] Some of the differences in splicing in cancerous cells may result from changes in phosphorylation of trans-acting splicing factors.[7] Others may be produced by changes in the relative amounts of splicing factors produced; for instance, breast cancer cells have been shown to have increased levels of the splicing factor SF2/ASF.[38] One study found that a relatively small percentage (383 out of over 26000) of alternative splicing variants were significantly higher in frequency in tumor cells than normal cells, suggesting that there is a limited set of genes which, when mis-spliced, contribute to tumor development.[39] It is believed however that the deleterious effects of mis-spliced transcripts are usually safeguarded and eliminated by a cellular posttranscriptional quality control mechanism termed Nonsense-mediated mRNA decay [NMD].[8]
One example of a specific splicing variant associated with cancers is in one of the human DNMT genes. Three DNMT genes encode enzymes that add methyl groups to DNA, a modification that often has regulatory effects. Several abnormally spliced DNMT3B mRNAs are found in tumors and cancer cell lines. In two separate studies, expression of two of these abnormally spliced mRNAs in mammalian cells caused changes in the DNA methylation patterns in those cells. Cells with one of the abnormal mRNAs also grew twice as fast as control cells, indicating a direct contribution to tumor development by this product.[7]
Another example is the Ron (MST1R) proto-oncogene. An important property of cancerous cells is their ability to move and invade normal tissue. Production of an abnormally spliced transcript of Ron has been found to be associated with increased levels of the SF2/ASF in breast cancer cells. The abnomal isoform of the Ron protein encoded by this mRNA leads to cell motility.[38]
Recent provocative studies point to a key function of chromatin structure and histone modifications in alternative splicing regulation. These insights suggest that epigenetic regulation determines not only what parts of the genome are expressed but also how they are spliced.[40]
Genome-wide analysis of alternative splicing
Genome-wide analysis of alternative splicing is a challenging task. Typically, alternatively spliced transcripts have been found by comparing EST sequences, but this requires sequencing of very large numbers of ESTs. Most EST libraries come from a very limited number of tissues, so tissue-specific splice variants are likely to be missed in any case. High-throughput approaches to investigate splicing fall into three categories; DNA microarray-based analyses, CLIP, and in vivo reporter gene assays.[21]
In microarray analysis, arrays of DNA fragments representing individual exons (e.g. Affymetrix exon microarray) or exon/exon boundaries (e.g. arrays from ExonHit or Jivan) have been used. The array is then probed with labeled cDNA from tissues of interest. The probe cDNAs bind to DNA from the exons that are included in mRNAs in their tissue of origin, or to DNA from the boundary where two exons have been joined. This can reveal the presence of particular alternatively spliced mRNAs.[2] Deep sequencing technologies are also being used to perform genome-wide studies of transcript variation.
CLIP (Cross-linking and immunoprecipitation) uses UV radiation to link proteins to RNA molecules in a tissue during splicing. A trans-acting splicing regulatory protein of interest is then precipitated using specific antibodies. When the RNA attached to that protein is isolated and cloned, it reveals the target sequences for that protein.[4]
Finally, it is possible to find the splicing proteins involved in a specific alternative splicing event by constructing reporter genes that will express one of two different fluorescent proteins depending on the splicing reaction that occurs. This method has been used to isolate mutants affecting splicing and thus to identify novel splicing regulatory proteins inactivated in those mutants.[4]
References
- ^ a b c d e f g h i j Black, Douglas L. (2003). "Mechanisms of alternative pre-messenger RNA splicing". Annual Reviews of Biochemistry 72 (1): 291–336. doi:10.1146/annurev.biochem.72.121801.161720. PMID 12626338.
- ^ a b c Pan, Q; Shai O, Lee LJ, Frey BJ, Blencowe BJ (Dec 2008). "Deep surveying of alternative splicing complexity in the human transcriptome by high-throughput sequencing". Nature Genetics 40 (12): 1413–1415. doi:10.1038/ng.259. PMID 18978789.
- ^ a b c d e f g h i j Matlin, AJ; Clark F, Smith, CWJ (May 2005). "Understanding alternative splicing: towards a cellular code". Nature Reviews 6 (5): 386–398. doi:10.1038/nrm1645. PMID 15956978.
- ^ a b c David, C. J.; Manley, J. L. (2008). "The search for alternative splicing regulators: new approaches offer a path to a splicing code". Genes & Development 22 (3): 279–85. doi:10.1101/gad.1643108. PMC 2731647. PMID 18245441. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2731647.
- ^ a b Skotheim, R I and Nees, M (2007). "Alternative splicing in cancer: noise, functional, or systematic?". The international journal of biochemistry & cell biology 39 (7-8): 1432–49. doi:10.1016/j.biocel.2007.02.016. PMID 17416541.
- ^ a b Bauer, Joseph Alan; He, Chunjiang; Zhou, Fang; Zuo, Zhixiang; Cheng, Hanhua; Zhou, Rongjia (2009). Bauer, Joseph Alan. ed. "A Global View of Cancer-Specific Transcript Variants by Subtractive Transcriptome-Wide Analysis". PLoS ONE 4 (3): e4732. Bibcode 2009PLoSO...4.4732H. doi:10.1371/journal.pone.0004732. PMC 2648985. PMID 19266097. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2648985.
- ^ a b c d Fackenthal, Jd; Godley, La (2008). "Aberrant RNA splicing and its functional consequences in cancer cells" (Free full text). Disease models & mechanisms 1 (1): 37–42. doi:10.1242/dmm.000331. PMC 2561970. PMID 19048051. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2561970.
- ^ a b Danckwardt S, Neu-Yilik G, Thermann R, Frede U, Hentze MW, Kulozik AE (2002). "Abnormally spliced beta-globin mRNAs: a single point mutation generates transcripts sensitive and insensitive to nonsense-mediated mRNA decay". Blood 99 (5): 1811–6. doi:10.1182/blood.V99.5.1811. PMID 11861299. http://bloodjournal.hematologylibrary.org/cgi/content/full/99/5/1811.
- ^ Chow LT, Gelinas RE, Broker TR, Roberts RJ (1977). "An amazing sequence arrangement at the 5' ends of adenovirus 2 messenger RNA". Cell 12 (1): 1–8. doi:10.1016/0092-8674(77)90180-5. PMID 902310.
- ^ Berget SM, Moore C, Sharp PA (1977). "Spliced segments at the 5' terminus of adenovirus 2 late mRNA". Proc. Natl. Acad. Sci. U.S.A. 74 (8): 3171–5. doi:10.1073/pnas.74.8.3171. PMC 431482. PMID 269380. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=431482.
- ^ a b c Leff SE, Rosenfeld MG, Evans RM (1986). "Complex transcriptional units: diversity in gene expression by alternative RNA processing". Annu. Rev. Biochem. 55 (1): 1091–117. doi:10.1146/annurev.bi.55.070186.005303. PMID 3017190.
- ^ Chow LT, Broker TR (1978). "The spliced structures of adenovirus 2 fiber message and the other late mRNAs". Cell 15 (2): 497–510. doi:10.1016/0092-8674(78)90019-3. PMID 719751.
- ^ Nevins JR, Darnell JE (1978). "Steps in the processing of Ad2 mRNA: poly(A)+ nuclear sequences are conserved and poly(A) addition precedes splicing". Cell 15 (4): 1477–93. doi:10.1016/0092-8674(78)90071-5. PMID 729004.
- ^ Rosenfeld MG, Amara SG, Roos BA, Ong ES, Evans RM (1981). "Altered expression of the calcitonin gene associated with RNA polymorphism". Nature 290 (5801): 63–5. doi:10.1038/290063a0. PMID 7207587.
- ^ Rosenfeld MG, Lin CR, Amara SG, et al (1982). "Calcitonin mRNA polymorphism: peptide switching associated with alternative RNA splicing events". Proc. Natl. Acad. Sci. U.S.A. 79 (6): 1717–21. doi:10.1073/pnas.79.6.1717. PMC 346051. PMID 6952224. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=346051.
- ^ Maki R, Roeder W, Traunecker A, et al (1981). "The role of DNA rearrangement and alternative RNA processing in the expression of immunoglobulin delta genes". Cell 24 (2): 353–65. doi:10.1016/0092-8674(81)90325-1. PMID 6786756.
- ^ Schmucker D, Clemens JC, Shu H, Worby CA, Xiao J, Muda M, Dixon JE, Zipursky SL (2000). "Drosophila Dscam is an axon guidance receptor exhibiting extraordinary molecular diversity". Cell 101 (6): 671–84. doi:10.1016/S0092-8674(00)80878-8. PMID 10892653.
- ^ a b c d Michael Sammeth; Sylvain Foissac; Roderic Guigó (2008). Brent, Michael R.. ed. "A general definition and nomenclature for alternative splicing events". PLoS Comput Biol. 4 (8): e1000147. doi:10.1371/journal.pcbi.1000147. PMC 2467475. PMID 18688268. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2467475.
- ^ Gao, K.; Masuda, A.; Matsuura, T.; Ohno, K. (2008). "Human branch point consensus sequence is yUnAy". Nucleic Acids Research 36 (7): 2257–67. doi:10.1093/nar/gkn073. PMC 2367711. PMID 18285363. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2367711.
- ^ Clark, David (2005). Molecular biology. Amsterdam: Elsevier Academic Press. ISBN 0-12-175551-7.
- ^ a b c d Wang, Z; Burge, Cb (2008). "Splicing regulation: from a parts list of regulatory elements to an integrated splicing code" (Free full text). RNA 14 (5): 802–13. doi:10.1261/rna.876308. PMC 2327353. PMID 18369186. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2327353.
- ^ a b Barash, Y; et al (2010). "Deciphering the splicing code". Nature 465 (7294): 53–59. doi:10.1038/nature09000. PMID 20445623.
- ^ Ke S, Zhang XH, Chasin LA (2008). "Positive selection acting on splicing motifs reflects compensatory evolution". Genome Res. 18 (4): 533–43. doi:10.1101/gr.070268.107. PMC 2279241. PMID 18204002. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2279241.
- ^ a b Lynch KW, Maniatis T (1996). "Assembly of specific SR protein complexes on distinct regulatory elements of the Drosophila doublesex splicing enhancer". Genes Dev. 10 (16): 2089–101. doi:10.1101/gad.10.16.2089. PMID 8769651.
- ^ Graveley BR, Hertel KJ, Maniatis T (2001). "The role of U2AF35 and U2AF65 in enhancer-dependent splicing". RNA 7 (6): 806–18. doi:10.1017/S1355838201010317. PMC 1370132. PMID 11421359. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=1370132.
- ^ Filipowicz, Ewa; Adegboyega, P.; Sanchez, R. L.; Gatalica, Zoran (2002). "Expression of CD95 (Fas) in sun-exposed human skin and cutaneous carcinomas". Cancer 94 (3): 814–9. doi:10.1002/cncr.10277. PMID 11857317.
- ^ a b Izquierdo JM, Majós N, Bonnal S, et al (2005). "Regulation of Fas alternative splicing by antagonistic effects of TIA-1 and PTB on exon definition". Mol. Cell 19 (4): 475–84. doi:10.1016/j.molcel.2005.06.015. PMID 16109372.
- ^ a b Zahler, A. M.; Damgaard, CK; Kjems, J; Caputi, M (2003). "SC35 and Heterogeneous Nuclear Ribonucleoprotein A/B Proteins Bind to a Juxtaposed Exonic Splicing Enhancer/Exonic Splicing Silencer Element to Regulate HIV-1 tat Exon 2 Splicing". Journal of Biological Chemistry 279 (11): 10077–84. doi:10.1074/jbc.M312743200. PMID 14703516.
- ^ Jacquenet, S.; M�reau, A; Bilodeau, PS; Damier, L; Stoltzfus, CM; Branlant, C (2001). "A Second Exon Splicing Silencer within Human Immunodeficiency Virus Type 1 tat Exon 2 Represses Splicing of Tat mRNA and Binds Protein hnRNP H". Journal of Biological Chemistry 276 (44): 40464–75. doi:10.1074/jbc.M104070200. PMID 11526107.
- ^ "HHMI Bulletin September 2005: Alternative Splicing". www.hhmi.org. http://www.hhmi.org/bulletin/sept2005/features/splicing.html. Retrieved 2009-05-26.
- ^ Irimia, Manuel; Rukov, Jakob; Penny, David; Roy, Scott (2007). "Functional and evolutionary analysis of alternatively spliced genes is consistent with an early eukaryotic origin of alternative splicing". BMC Evolutionary Biology 7: 188. doi:10.1186/1471-2148-7-188. PMC 2082043. PMID 17916237. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2082043.
- ^ Ewing, B; Green P (June 2000). "Analysis of expressed sequence tags indicates 35,000 human genes". Nature Genetics 25 (2): 232–234. doi:10.1038/76115. PMID 10835644.
- ^ Crollius, HR; et al. (2000). "Estimate of human gene number provided by genome-wide analysis using Tetraodon nigroviridis DNA sequence". Nature Genetics 25 (2): 235–238. doi:10.1038/76118. PMID 10835645.
- ^ David Brett; Heike Pospisil; Juan Valcárcel; Jens Reich; Peer Bork (2002). "Alternative splicing and genome complexity". Nature Genetics 30 (1): 29–30. doi:10.1038/ng803. PMID 11743582.
- ^ Kim, E.; Magen, A.; Ast, G. (2006). "Different levels of alternative splicing among eukaryotes". Nucleic Acids Research 35 (1): 125–31. doi:10.1093/nar/gkl924. PMC 1802581. PMID 17158149. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=1802581.
- ^ L�pez-Bigas, Núria; Audit, Benjamin; Ouzounis, Christos; Parra, Genís; Guig�, Roderic (2005). "Are splicing mutations the most frequent cause of hereditary disease?". FEBS Letters 579 (9): 1900–3. doi:10.1016/j.febslet.2005.02.047. PMID 15792793.
- ^ Kim E, Goren A, Ast G (2008). "Insights into the connection between cancer and alternative splicing". Trends Genet. 24 (1): 7–10. doi:10.1016/j.tig.2007.10.001. PMID 18054115.
- ^ a b Ghigna C, Giordano S, Shen H, et al. (2005). "Cell motility is controlled by SF2/ASF through alternative splicing of the Ron proto-oncogene". Mol. Cell 20 (6): 881–90. doi:10.1016/j.molcel.2005.10.026. PMID 16364913.
- ^ Hui L, Zhang X, Wu X, et al. (2004). "Identification of alternatively spliced mRNA variants related to cancers by genome-wide ESTs alignment". Oncogene 23 (17): 3013–23. doi:10.1038/sj.onc.1207362. PMID 15048092.
- ^ Luco, RF; Allo, M; Schor, IE; Kornblihtt, AR; Misteli, T. (2011). "Epigenetics in alternative pre-mRNA splicing". Cell 144 (1): 16–26. doi:10.1016/j.cell.2010.11.056. PMC 3038581. PMID 21215366. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=3038581.
External links
- A General Definition and Nomenclature for Alternative Splicing Events at SciVee
- AStalavista (Alternative Splicing landscape visualization tool), a method for the computationally exhaustive classification of Alternative Splicing Structures
- Stamms-lab.net: Research Group dealing with alternative Splicing issues and mis-splicing in human diseases
- Alternative Splicing of ion channels in the brain, connected to mental and neurological diseases
- BIPASS: Web Services in Alternative Splicing
See also
- AspicDB database
- Alternative polyadenylation (section of Polyadenylation)
Nuclear Precursor mRNA · 5' cap formation · Polyadenylation (CPSF, CstF, PAP, PAB2, CFI, CFII) · Poly(A)-binding protein
RNA splicing: intron/exon · snRNP · spliceosome (minor spliceosome, U1) · alternative splicing · pre-mRNA processing factor (PLRG1, PRPF3, PRPF4, PRPF4B, PRPF6, PRPF8, PRPF18, PRPF19, PRPF31, PRPF38A, PRPF38B, PRPF39, PRPF40A, PRPF40B)
RNA editing · PolyuridylationCytosolic see also disorders of transcription and post transcriptional modification
B bsyn: dna (repl, cycl, reco, repr) · tscr (fact, tcrg, nucl, rnat, rept, ptts) · tltn (risu, pttl, nexn) · dnab, rnab/runp · stru (domn, 1°, 2°, 3°, 4°)Categories:
Wikimedia Foundation. 2010.